Targeting Extracellular Heat Shock Protein 70 Ameliorates Doxorubicin-Induced Heart Failure Through Resolution of Toll-Like Receptor 2-Mediated Myocardial Inflammation
- PMID: 31576776
- PMCID: PMC6818050
- DOI: 10.1161/JAHA.119.012338
Targeting Extracellular Heat Shock Protein 70 Ameliorates Doxorubicin-Induced Heart Failure Through Resolution of Toll-Like Receptor 2-Mediated Myocardial Inflammation
Abstract
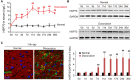
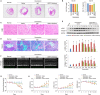
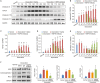
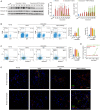
Background Heart failure (HF) is one of the most significant causes of morbidity and mortality for the cardiovascular risk population. We found previously that extracellular HSP70 (heat shock protein) is an important trigger in cardiac hypertrophy and fibrosis, which are associated with the development of heart dysfunction. However, the potential role of HSP70 in response to HF and whether it could be a target for the therapy of HF remain unknown. Methods and Results An HF mouse model was generated by a single IP injection of doxorubicin at a dose of 15 mg/kg. Ten days later, these mice were treated with an HSP70 neutralizing antibody for 5 times. We observed that doxorubicin treatment increased circulating HSP70 and expression of HSP70 in myocardium and promoted its extracellular release in the heart. Blocking extracellular HSP70 activity by its antibody significantly ameliorated doxorubicin-induced left ventricular dilation and dysfunction, which was accompanied by a significant inhibition of cardiac fibrosis. The cardioprotective effect of the anti-HSP70 antibody was largely attributed to its ability to promote the resolution of myocardial inflammation, as evidenced by its suppression of the toll-like receptor 2-associated signaling cascade and modulation of the intracellular distribution of the p50 and p65 subunits of nuclear factor-κB. Conclusions Extracellular HSP70 serves as a noninfectious inflammatory factor in the development of HF, and blocking extracellular HSP70 activity may provide potential therapeutic benefits for the treatment of HF.
Keywords: cardiac dysfunction; cardiac remodeling; damage‐associated molecular patterns; nuclear factor‐κB; toll‐like receptor.
Figures
References
-
- Braunwald E. The war against heart failure: the Lancet lecture. Lancet. 2015;385:812–824. - PubMed
-
- Mebazaa A, Yilmaz MB, Levy P, Ponikowski P, Peacock WF, Laribi S, Ristic AD, Lambrinou E, Masip J, Riley JP, McDonagh T, Mueller C, deFilippi C, Harjola VP, Thiele H, Piepoli MF, Metra M, Maggioni A, McMurray JJ, Dickstein K, Damman K, Seferovic PM, Ruschitzka F, Leite‐Moreira AF, Bellou A, Anker SD, Filippatos G. Recommendations on pre‐hospital and early hospital management of acute heart failure: a consensus paper from the Heart Failure Association of the European Society of Cardiology, the European Society of Emergency Medicine and the Society of Academic Emergency Medicine–short version. Eur Heart J. 2015;36:1958–1966. - PubMed
-
- Zhang Y, Bauersachs J, Langer HF. Immune mechanisms in heart failure. Eur J Heart Fail. 2017;19:1379–1389. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

