Effect of Postextubation High-Flow Nasal Oxygen With Noninvasive Ventilation vs High-Flow Nasal Oxygen Alone on Reintubation Among Patients at High Risk of Extubation Failure: A Randomized Clinical Trial
- PMID: 31577036
- PMCID: PMC6802261
- DOI: 10.1001/jama.2019.14901
Effect of Postextubation High-Flow Nasal Oxygen With Noninvasive Ventilation vs High-Flow Nasal Oxygen Alone on Reintubation Among Patients at High Risk of Extubation Failure: A Randomized Clinical Trial
Erratum in
-
Incomplete Intervention Description, Incorrect Exploratory Outcome Data, and Incorrect Axis Labels.JAMA. 2020 Feb 25;323(8):793. doi: 10.1001/jama.2020.0373. JAMA. 2020. PMID: 32096830 Free PMC article. No abstract available.
Abstract
Importance: High-flow nasal oxygen may prevent postextubation respiratory failure in the intensive care unit (ICU). The combination of high-flow nasal oxygen with noninvasive ventilation (NIV) may be an optimal strategy of ventilation to avoid reintubation.
Objective: To determine whether high-flow nasal oxygen with prophylactic NIV applied immediately after extubation could reduce the rate of reintubation, compared with high-flow nasal oxygen alone, in patients at high risk of extubation failure in the ICU.
Design, setting, and participants: Multicenter randomized clinical trial conducted from April 2017 to January 2018 among 641 patients at high risk of extubation failure (ie, older than 65 years or with an underlying cardiac or respiratory disease) at 30 ICUs in France; follow-up was until April 2018.
Interventions: Patients were randomly assigned to high-flow nasal oxygen alone (n = 306) or high-flow nasal oxygen alternating with NIV (n = 342) immediately after extubation.
Main outcomes and measures: The primary outcome was the proportion of patients reintubated at day 7; secondary outcomes included postextubation respiratory failure at day 7, reintubation rates up until ICU discharge, and ICU mortality.
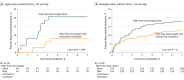
Results: Among 648 patients who were randomized (mean [SD] age, 70 [10] years; 219 women [34%]), 641 patients completed the trial. The reintubation rate at day 7 was 11.8% (95% CI, 8.4%-15.2%) (40/339) with high-flow nasal oxygen and NIV and 18.2% (95% CI, 13.9%-22.6%) (55/302) with high-flow nasal oxygen alone (difference, -6.4% [95% CI, -12.0% to -0.9%]; P = .02). Among the 11 prespecified secondary outcomes, 6 showed no significant difference. The proportion of patients with postextubation respiratory failure at day 7 (21% vs 29%; difference, -8.7% [95% CI, -15.2% to -1.8%]; P = .01) and reintubation rates up until ICU discharge (12% vs 20%, difference -7.4% [95% CI, -13.2% to -1.8%]; P = .009) were significantly lower with high-flow nasal oxygen and NIV than with high-flow nasal oxygen alone. ICU mortality rates were not significantly different: 6% with high-flow nasal oxygen and NIV and 9% with high-flow nasal oxygen alone (difference, -2.4% [95% CI, -6.7% to 1.7%]; P = .25).
Conclusions and relevance: In mechanically ventilated patients at high risk of extubation failure, the use of high-flow nasal oxygen with NIV immediately after extubation significantly decreased the risk of reintubation compared with high-flow nasal oxygen alone.
Trial registration: ClinicalTrials.gov Identifier: NCT03121482.
Conflict of interest statement
Figures

Comment in
-
Non-invasive ventilation and high-flow nasal oxygenation: Looking beyond extubation failure?Anaesth Crit Care Pain Med. 2019 Dec;38(6):583-584. doi: 10.1016/j.accpm.2019.10.010. Anaesth Crit Care Pain Med. 2019. PMID: 31785702 No abstract available.
-
Lower Reintubation Risk with Noninvasive Ventilation Plus High-Flow Nasal Oxygen.Am J Nurs. 2020 Feb;120(2):50. doi: 10.1097/01.NAJ.0000654332.96458.c4. Am J Nurs. 2020. PMID: 31977421 No abstract available.
-
Strategies to Avoid Extubation Failure Among ICU Patients.JAMA. 2020 Mar 3;323(9):891-892. doi: 10.1001/jama.2019.21951. JAMA. 2020. PMID: 32125393 No abstract available.
-
Strategies for Liberation from Mechanical Ventilation.Am J Respir Crit Care Med. 2021 May 1;203(9):1183-1185. doi: 10.1164/rccm.202006-2312RR. Am J Respir Crit Care Med. 2021. PMID: 33631088 No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

