Changes in End-of-Life Practices in European Intensive Care Units From 1999 to 2016
- PMID: 31577037
- PMCID: PMC6777263
- DOI: 10.1001/jama.2019.14608
Changes in End-of-Life Practices in European Intensive Care Units From 1999 to 2016
Erratum in
-
Omitted Author Contributions.JAMA. 2019 Nov 5;322(17):1718. doi: 10.1001/jama.2019.17746. JAMA. 2019. PMID: 31688867 Free PMC article. No abstract available.
Abstract
Importance: End-of-life decisions occur daily in intensive care units (ICUs) around the world, and these practices could change over time.
Objective: To determine the changes in end-of-life practices in European ICUs after 16 years.
Design, setting, and participants: Ethicus-2 was a prospective observational study of 22 European ICUs previously included in the Ethicus-1 study (1999-2000). During a self-selected continuous 6-month period at each ICU, consecutive patients who died or had any limitation of life-sustaining therapy from September 2015 until October 2016 were included. Patients were followed up until death or until 2 months after the first treatment limitation decision.
Exposures: Comparison between the 1999-2000 cohort vs 2015-2016 cohort.
Main outcomes and measures: End-of-life outcomes were classified into 5 mutually exclusive categories (withholding of life-prolonging therapy, withdrawing of life-prolonging therapy, active shortening of the dying process, failed cardiopulmonary resuscitation [CPR], brain death). The primary outcome was whether patients received any treatment limitations (withholding or withdrawing of life-prolonging therapy or shortening of the dying process). Outcomes were determined by senior intensivists.
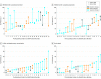
Results: Of 13 625 patients admitted to participating ICUs during the 2015-2016 study period, 1785 (13.1%) died or had limitations of life-prolonging therapies and were included in the study. Compared with the patients included in the 1999-2000 cohort (n = 2807), the patients in 2015-2016 cohort were significantly older (median age, 70 years [interquartile range {IQR}, 59-79] vs 67 years [IQR, 54-75]; P < .001) and the proportion of female patients was similar (39.6% vs 38.7%; P = .58). Significantly more treatment limitations occurred in the 2015-2016 cohort compared with the 1999-2000 cohort (1601 [89.7%] vs 1918 [68.3%]; difference, 21.4% [95% CI, 19.2% to 23.6%]; P < .001), with more withholding of life-prolonging therapy (892 [50.0%] vs 1143 [40.7%]; difference, 9.3% [95% CI, 6.4% to 12.3%]; P < .001), more withdrawing of life-prolonging therapy (692 [38.8%] vs 695 [24.8%]; difference, 14.0% [95% CI, 11.2% to 16.8%]; P < .001), less failed CPR (110 [6.2%] vs 628 [22.4%]; difference, -16.2% [95% CI, -18.1% to -14.3%]; P < .001), less brain death (74 [4.1%] vs 261 [9.3%]; difference, -5.2% [95% CI, -6.6% to -3.8%]; P < .001) and less active shortening of the dying process (17 [1.0%] vs 80 [2.9%]; difference, -1.9% [95% CI, -2.7% to -1.1%]; P < .001).
Conclusions and relevance: Among patients who had treatment limitations or died in 22 European ICUs in 2015-2016, compared with data reported from the same ICUs in 1999-2000, limitations in life-prolonging therapies occurred significantly more frequently and death without limitations in life-prolonging therapies occurred significantly less frequently. These findings suggest a shift in end-of-life practices in European ICUs, but the study is limited in that it excluded patients who survived ICU hospitalization without treatment limitations.
Conflict of interest statement
Figures
References
-
- Sprung CL, Truog RD, Curtis JR, et al. . Seeking worldwide professional consensus on the principles of end-of-life care for the critically ill: the Consensus for Worldwide End-of-Life Practice for Patients in Intensive Care Units (WELPICUS) study. Am J Respir Crit Care Med. 2014;190(8):855-866. doi:10.1164/rccm.201403-0593CC - DOI - PubMed
-
- Council of Europe Guide on the decision-making process regarding medical treatment in end-of-life situations. https://www.coe.int/t/dg3/healthbioethic/conferences_and_symposia/Guide%.... Accessed August 30, 2018.
LinkOut - more resources
Full Text Sources
Medical

