Ventilation/Perfusion Matching: Of Myths, Mice, and Men
- PMID: 31577170
- PMCID: PMC7002871
- DOI: 10.1152/physiol.00016.2019
Ventilation/Perfusion Matching: Of Myths, Mice, and Men
Abstract
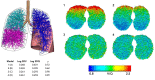
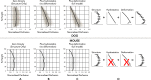
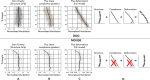
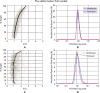
Despite a huge range in lung size between species, there is little measured difference in the ability of the lung to provide a well-matched air flow (ventilation) to blood flow (perfusion) at the gas exchange tissue. Here, we consider the remarkable similarities in ventilation/perfusion matching between species through a biophysical lens and consider evidence that matching in large animals is dominated by gravity but in small animals by structure.
Keywords: computational models; lungs; perfusion; ventilation.
Figures
Similar articles
-
Gravity outweighs the contribution of structure to passive ventilation-perfusion matching in the supine adult human lung.J Appl Physiol (1985). 2018 Jan 1;124(1):23-33. doi: 10.1152/japplphysiol.00791.2016. Epub 2017 Oct 19. J Appl Physiol (1985). 2018. PMID: 29051337 Free PMC article.
-
Microgravity and the respiratory system.Eur Respir J. 2014 May;43(5):1459-71. doi: 10.1183/09031936.00001414. Epub 2014 Mar 6. Eur Respir J. 2014. PMID: 24603820 Review.
-
Distribution of blood flow and ventilation in the lung: gravity is not the only factor.Br J Anaesth. 2007 Apr;98(4):420-8. doi: 10.1093/bja/aem036. Epub 2007 Mar 8. Br J Anaesth. 2007. PMID: 17347182 Review.
-
Gas exchange under altered gravitational stress.Compr Physiol. 2011 Jan;1(1):339-55. doi: 10.1002/cphy.c090007. Compr Physiol. 2011. PMID: 23737176 Review.
-
Spatial distribution of ventilation and perfusion: mechanisms and regulation.Compr Physiol. 2011 Jan;1(1):375-95. doi: 10.1002/cphy.c100002. Compr Physiol. 2011. PMID: 23737178 Review.
Cited by
-
Roadmap for an imaging and modelling paediatric study in rural NZ.Front Physiol. 2023 Mar 10;14:1104838. doi: 10.3389/fphys.2023.1104838. eCollection 2023. Front Physiol. 2023. PMID: 36969588 Free PMC article.
-
Breathing patterns and associated cardiovascular changes in intermittently breathing animals: (Partially) correcting a semantic quagmire.Exp Physiol. 2024 Jul;109(7):1051-1065. doi: 10.1113/EP091784. Epub 2024 Mar 19. Exp Physiol. 2024. PMID: 38502538 Free PMC article. Review.
-
3D Magnetic Resonance Spirometry.Sci Rep. 2020 Jun 15;10(1):9649. doi: 10.1038/s41598-020-66202-7. Sci Rep. 2020. PMID: 32541799 Free PMC article.
-
Three-Dimensional Whole-Organ Characterization of the Regional Alveolar Morphology in Normal Murine Lungs.Front Physiol. 2021 Dec 8;12:755468. doi: 10.3389/fphys.2021.755468. eCollection 2021. Front Physiol. 2021. PMID: 34955878 Free PMC article.
-
Evaluation of Different Contrast Agents for Regional Lung Perfusion Measurement Using Electrical Impedance Tomography: An Experimental Pilot Study.J Clin Med. 2023 Apr 7;12(8):2751. doi: 10.3390/jcm12082751. J Clin Med. 2023. PMID: 37109088 Free PMC article.
References
-
- Asadi AK, Sá RC, Kim NH, Theilmann RJ, Hopkins SR, Buxton RB, Prisk GK. Inhaled nitric oxide alters the distribution of blood flow in the healthy human lung, suggesting active hypoxic pulmonary vasoconstriction in normoxia. J Appl Physiol (1985) 118: 331–343, 2015. doi:10.1152/japplphysiol.01354.2013. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

