Isoniazid Preventive Therapy in HIV-Infected Pregnant and Postpartum Women
- PMID: 31577875
- PMCID: PMC7051859
- DOI: 10.1056/NEJMoa1813060
Isoniazid Preventive Therapy in HIV-Infected Pregnant and Postpartum Women
Abstract
Background: The safety, efficacy, and appropriate timing of isoniazid therapy to prevent tuberculosis in pregnant women with human immunodeficiency virus (HIV) infection who are receiving antiretroviral therapy are unknown.
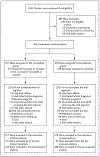
Methods: In this multicenter, double-blind, placebo-controlled, noninferiority trial, we randomly assigned pregnant women with HIV infection to receive isoniazid preventive therapy for 28 weeks, initiated either during pregnancy (immediate group) or at week 12 after delivery (deferred group). Mothers and infants were followed through week 48 after delivery. The primary outcome was a composite of treatment-related maternal adverse events of grade 3 or higher or permanent discontinuation of the trial regimen because of toxic effects. The noninferiority margin was an upper boundary of the 95% confidence interval for the between-group difference in the rate of the primary outcome of less than 5 events per 100 person-years.
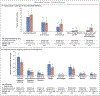
Results: A total of 956 women were enrolled. A primary outcome event occurred in 72 of 477 women (15.1%) in the immediate group and in 73 of 479 (15.2%) in the deferred group (incidence rate, 15.03 and 14.93 events per 100 person-years, respectively; rate difference, 0.10; 95% confidence interval [CI], -4.77 to 4.98, which met the criterion for noninferiority). Two women in the immediate group and 4 women in the deferred group died (incidence rate, 0.40 and 0.78 per 100 person-years, respectively; rate difference, -0.39; 95% CI, -1.33 to 0.56); all deaths occurred during the postpartum period, and 4 were from liver failure (2 of the women who died from liver failure had received isoniazid [1 in each group]). Tuberculosis developed in 6 women (3 in each group); the incidence rate was 0.60 per 100 person-years in the immediate group and 0.59 per 100 person-years in the deferred group (rate difference, 0.01; 95% CI, -0.94 to 0.96). There was a higher incidence in the immediate group than in the deferred group of an event included in the composite adverse pregnancy outcome (stillbirth or spontaneous abortion, low birth weight in an infant, preterm delivery, or congenital anomalies in an infant) (23.6% vs. 17.0%; difference, 6.7 percentage points; 95% CI, 0.8 to 11.9).
Conclusions: The risks associated with initiation of isoniazid preventive therapy during pregnancy appeared to be greater than those associated with initiation of therapy during the postpartum period. (Funded by the National Institutes of Health; IMPAACT P1078 TB APPRISE ClinicalTrials.gov number, NCT01494038.).
Copyright © 2019 Massachusetts Medical Society.
Figures
Comment in
-
HIV, Pregnancy, and Isoniazid Preventive Therapy.N Engl J Med. 2020 Mar 19;382(12):1184. doi: 10.1056/NEJMc1916664. N Engl J Med. 2020. PMID: 32187482 No abstract available.
References
-
- Global tuberculosis report 2018. Geneva: World Health Organization, 2018. (http://www.who.int/tb/publications/global_report/en/).
-
- Jana N, Vasishta K, Saha SC, Ghosh K. Obstetrical outcomes among women with extrapulmonary tuberculosis. N Engl J Med 1999;341:645–9. - PubMed
-
- Pillay T, Khan M, Moodley J, Adhikari M, Coovadia H. Perinatal tuberculosis and HIV-1: considerations for resource-limited settings. Lancet Infect Dis 2004;4:155–65. - PubMed
-
- Gomes VF, Andersen A, Wejse C, et al. Impact of tuberculosis exposure at home on mortality in children under 5 years of age in Guinea-Bissau. Thorax 2011;66:163–7. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- HHSN275201800001C/HD/NICHD NIH HHS/United States
- UM1 AI068632/AI/NIAID NIH HHS/United States
- UM1 AI069453/AI/NIAID NIH HHS/United States
- UM1 AI068616/AI/NIAID NIH HHS/United States
- U01 AI069521/AI/NIAID NIH HHS/United States
- Overall support for the International Maternal Pe/NH/NIH HHS/United States
- UM1 AI069436/AI/NIAID NIH HHS/United States
- HHSN275201800001I/HD/NICHD NIH HHS/United States
- U01 AI068632/AI/NIAID NIH HHS/United States
- R21 MH083308/MH/NIMH NIH HHS/United States
- UM1 AI069521/AI/NIAID NIH HHS/United States
- UM1 AI069465/AI/NIAID NIH HHS/United States
- UM1 AI106716/AI/NIAID NIH HHS/United States
- U01 AI069436/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
