AAV2-BDNF promotes respiratory axon plasticity and recovery of diaphragm function following spinal cord injury
- PMID: 31577916
- PMCID: PMC6894085
- DOI: 10.1096/fj.201901730R
AAV2-BDNF promotes respiratory axon plasticity and recovery of diaphragm function following spinal cord injury
Abstract
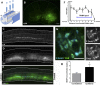
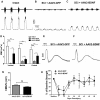
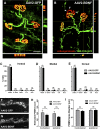
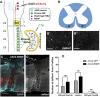
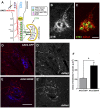
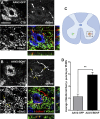
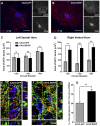
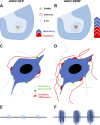
More than half of spinal cord injury (SCI) cases occur in the cervical region, leading to respiratory dysfunction due to damaged neural circuitry that controls critically important muscles such as the diaphragm. The C3-C5 spinal cord is the location of phrenic motor neurons (PhMNs) that are responsible for diaphragm activation; PhMNs receive bulbospinal excitatory drive predominately from supraspinal neurons of the rostral ventral respiratory group (rVRG). Cervical SCI results in rVRG axon damage, PhMN denervation, and consequent partial-to-complete paralysis of hemidiaphragm. In a rat model of C2 hemisection SCI, we expressed the axon guidance molecule, brain-derived neurotrophic factor (BDNF), selectively at the location of PhMNs (ipsilateral to lesion) to promote directed growth of rVRG axons toward PhMN targets by performing intraspinal injections of adeno-associated virus serotype 2 (AAV2)-BDNF vector. AAV2-BDNF promoted significant functional diaphragm recovery, as assessed by in vivo electromyography. Within the PhMN pool ipsilateral to injury, AAV2-BDNF robustly increased sprouting of both spared contralateral-originating rVRG axons and serotonergic fibers. Furthermore, AAV2-BDNF significantly increased numbers of putative monosynaptic connections between PhMNs and these sprouting rVRG and serotonergic axons. These findings show that targeting circuit plasticity mechanisms involving the enhancement of synaptic inputs from spared axon populations is a powerful strategy for restoring respiratory function post-SCI.-Charsar, B. A., Brinton, M. A., Locke, K., Chen, A. Y., Ghosh, B., Urban, M. W., Komaravolu, S., Krishnamurthy, K., Smit, R., Pasinelli, P., Wright, M. C., Smith, G. M., Lepore, A. C. AAV2-BDNF promotes respiratory axon plasticity and recovery of diaphragm function following spinal cord injury.
Keywords: SCI; cervical; phrenic; sprouting.
Conflict of interest statement
The authors thank Dr. Rich Smeyne (Thomas Jefferson University) for sharing his expertise in 2-dimensional stereology counting techniques, and for his help with implementing these methods, and Dr. Robert Sterling (Thomas Jefferson University) for his patience and assistance with statistical analysis. This work was supported by the U.S. National Institutes of Health, National Institute of Neurological Disorders and Stroke (NINDS; 2R01NS079702 to A.C.L. and 1F30NS103436 to B.A.C.), the Craig H. Neilsen Foundation (476686 to A.C.L.), and the Shriners Hospitals for Children Viral Core Grant SHC 84051 (to G.M.S.). The authors declare no conflicts of interest.
Figures
References
-
- Tator C. H., Fehlings M. G. (1991) Review of the secondary injury theory of acute spinal cord trauma with emphasis on vascular mechanisms. J. Neurosurg. 75, 15–26 - PubMed
-
- Park E., Velumian A. A., Fehlings M. G. (2004) The role of excitotoxicity in secondary mechanisms of spinal cord injury: a review with an emphasis on the implications for white matter degeneration. J. Neurotrauma 21, 754–774 - PubMed
-
- Dobbins E. G., Feldman J. L. (1994) Brainstem network controlling descending drive to phrenic motoneurons in rat. J. Comp. Neurol. 347, 64–86 - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

