Discovering Cross-Reactivity in Urine Drug Screening Immunoassays through Large-Scale Analysis of Electronic Health Records
- PMID: 31578215
- PMCID: PMC7055671
- DOI: 10.1373/clinchem.2019.305409
Discovering Cross-Reactivity in Urine Drug Screening Immunoassays through Large-Scale Analysis of Electronic Health Records
Abstract
Background: Exposure to drugs of abuse is frequently assessed using urine drug screening (UDS) immunoassays. Although fast and relatively inexpensive, UDS assays often cross-react with unrelated compounds, which can lead to false-positive results and impair patient care. The current process of identifying cross-reactivity relies largely on case reports, making it sporadic and inefficient, and rendering knowledge of cross-reactivity incomplete. Here, we present a systematic approach to discover cross-reactive substances using data from electronic health records (EHRs).
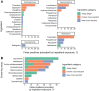
Methods: Using our institution's EHR data, we assembled a data set of 698651 UDS results across 10 assays and linked each UDS result to the corresponding individual's previous medication exposures. We hypothesized that exposure to a cross-reactive ingredient would increase the odds of a false-positive screen. For 2201 assay-ingredient pairs, we quantified potential cross-reactivity as an odds ratio from logistic regression. We then evaluated cross-reactivity experimentally by spiking the ingredient or its metabolite into drug-free urine and testing the spiked samples on each assay.
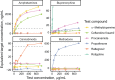
Results: Our approach recovered multiple known cross-reactivities. After accounting for concurrent exposures to multiple ingredients, we selected 18 compounds (13 parent drugs and 5 metabolites) to evaluate experimentally. We validated 12 of 13 tested assay-ingredient pairs expected to show cross-reactivity by our analysis, discovering previously unknown cross-reactivities affecting assays for amphetamines, buprenorphine, cannabinoids, and methadone.
Conclusions: Our findings can help laboratorians and providers interpret presumptive positive UDS results. Our data-driven approach can serve as a model for high-throughput discovery of substances that interfere with laboratory tests.
© 2019 American Association for Clinical Chemistry.
Figures

Comment in
-
Answering Unanswerable Questions in the Clinical Laboratory with Data Warehouses.Clin Chem. 2019 Dec;65(12):1471-1473. doi: 10.1373/clinchem.2019.311654. Epub 2019 Oct 18. Clin Chem. 2019. PMID: 31628137 No abstract available.
-
Pitfalls in Answering Questions in the Laboratory with Data Warehouses.Clin Chem. 2020 Jul 1;66(7):983-985. doi: 10.1093/clinchem/hvaa109. Clin Chem. 2020. PMID: 32556310 No abstract available.
Similar articles
-
Analysis of Electronic Health Records Reveals Medication-Related Interference on Point-of-Care Urine Drug Screening Assays.J Anal Toxicol. 2022 Feb 14;46(1):99-102. doi: 10.1093/jat/bkaa179. J Anal Toxicol. 2022. PMID: 33216907 Free PMC article.
-
A Computational Approach to Identify Interfering Medications on Urine Drug Screening Assays without Data from Confirmatory Testing.J Anal Toxicol. 2021 Apr 12;45(4):325-330. doi: 10.1093/jat/bkaa140. J Anal Toxicol. 2021. PMID: 32991692 Free PMC article.
-
False-positive interferences of common urine drug screen immunoassays: a review.J Anal Toxicol. 2014 Sep;38(7):387-96. doi: 10.1093/jat/bku075. Epub 2014 Jul 1. J Anal Toxicol. 2014. PMID: 24986836 Review.
-
Accidental intoxications in toddlers: lack of cross-reactivity of vilazodone and its urinary metabolite M17 with drug of abuse screening immunoassays.BMC Clin Pathol. 2019 Feb 18;19:2. doi: 10.1186/s12907-019-0084-9. eCollection 2019. BMC Clin Pathol. 2019. PMID: 30820187 Free PMC article.
-
Psychiatric and non-psychiatric drugs causing false-positive amphetamines urine test in psychiatric patients: a pharmacovigilance analysis using FAERS.Expert Rev Clin Pharmacol. 2023 May;16(5):453-465. doi: 10.1080/17512433.2023.2211261. Epub 2023 May 15. Expert Rev Clin Pharmacol. 2023. PMID: 37147189 Review.
Cited by
-
Applicability of a Chemiluminescence Immunoassay to Screen Postmortem Bile Specimens and Its Agreement with Confirmation Analysis.Int J Mol Sci. 2024 Mar 29;25(7):3825. doi: 10.3390/ijms25073825. Int J Mol Sci. 2024. PMID: 38612632 Free PMC article.
-
Analysis of Electronic Health Records Reveals Medication-Related Interference on Point-of-Care Urine Drug Screening Assays.J Anal Toxicol. 2022 Feb 14;46(1):99-102. doi: 10.1093/jat/bkaa179. J Anal Toxicol. 2022. PMID: 33216907 Free PMC article.
-
A Computational Approach to Identify Interfering Medications on Urine Drug Screening Assays without Data from Confirmatory Testing.J Anal Toxicol. 2021 Apr 12;45(4):325-330. doi: 10.1093/jat/bkaa140. J Anal Toxicol. 2021. PMID: 32991692 Free PMC article.
-
Bringing the clinical laboratory into the strategy to advance diagnostic excellence.Diagnosis (Berl). 2021 Jan 6;8(3):281-294. doi: 10.1515/dx-2020-0119. Print 2021 Aug 26. Diagnosis (Berl). 2021. PMID: 33554526 Free PMC article. Review.
-
Automatic Estimation of the Most Likely Drug Combination in Electronic Health Records Using the Smooth Algorithm: Development and Validation Study.JMIR Med Inform. 2022 Nov 15;10(11):e37976. doi: 10.2196/37976. JMIR Med Inform. 2022. PMID: 36378514 Free PMC article.
References
-
- Saitman A, Park H-D, Fitzgerald RL. False-positive interferences of common urine drug screen immunoassays: a review. J Anal Toxicol 2014;38:387–96. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

