Tumor Mutational Burden as a Predictive Biomarker for Response to Immune Checkpoint Inhibitors: A Review of Current Evidence
- PMID: 31578273
- PMCID: PMC6964127
- DOI: 10.1634/theoncologist.2019-0244
Tumor Mutational Burden as a Predictive Biomarker for Response to Immune Checkpoint Inhibitors: A Review of Current Evidence
Abstract
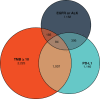
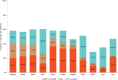
Treatment with immune checkpoint inhibitors (ICPIs) extends survival in a proportion of patients across multiple cancers. Tumor mutational burden (TMB)-the number of somatic mutations per DNA megabase (Mb)-has emerged as a proxy for neoantigen burden that is an independent biomarker associated with ICPI outcomes. Based on findings from recent studies, TMB can be reliably estimated using validated algorithms from next-generation sequencing assays that interrogate a sufficiently large subset of the exome as an alternative to whole-exome sequencing. Biological processes contributing to elevated TMB can result from exposure to cigarette smoke and ultraviolet radiation, from deleterious mutations in mismatch repair leading to microsatellite instability, or from mutations in the DNA repair machinery. A variety of clinical studies have shown that patients with higher TMB experience longer survival and greater response rates following treatment with ICPIs compared with those who have lower TMB levels; this includes a prospective randomized clinical trial that found a TMB threshold of ≥10 mutations per Mb to be predictive of longer progression-free survival in patients with non-small cell lung cancer. Multiple trials are underway to validate the predictive values of TMB across cancer types and in patients treated with other immunotherapies. Here we review the rationale, algorithm development methodology, and existing clinical data supporting the use of TMB as a predictive biomarker for treatment with ICPIs. We discuss emerging roles for TMB and its potential future value for stratifying patients according to their likelihood of ICPI treatment response. IMPLICATIONS FOR PRACTICE: Tumor mutational burden (TMB) is a newly established independent predictor of immune checkpoint inhibitor (ICPI) treatment outcome across multiple tumor types. Certain next-generation sequencing-based techniques allow TMB to be reliably estimated from a subset of the exome without the use of whole-exome sequencing, thus facilitating the adoption of TMB assessment in community oncology settings. Analyses of multiple clinical trials across several cancer types have demonstrated that TMB stratifies patients who are receiving ICPIs by response rate and survival. TMB, alongside other genomic biomarkers, may provide complementary information in selecting patients for ICPI-based therapies.
Keywords: Antibodies/therapeutic use; DNA; DNA mutational analysis; Genes; Neoplasm; Programmed cell death 1 receptor; Sequence analysis.
© AlphaMed Press 2019.
Conflict of interest statement
Figures


References
-
- Garon EB, Rizvi NA, Hui R et al. Pembrolizumab for the treatment of non‐small‐cell lung cancer. N Engl J Med 2015;372:2018–2028. - PubMed
-
- Fehrenbacher L, Spira A, Ballinger M et al. Atezolizumab versus docetaxel for patients with previously treated non‐small‐cell lung cancer (POPLAR): A multicentre, open‐label, phase 2 randomised controlled trial. Lancet 2016;387:1837–1846. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

