JARID2 and the PRC2 complex regulate the cell cycle in skeletal muscle
- PMID: 31578284
- PMCID: PMC6926451
- DOI: 10.1074/jbc.RA119.010060
JARID2 and the PRC2 complex regulate the cell cycle in skeletal muscle
Abstract
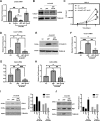
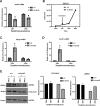
JARID2 is a noncatalytic member of the polycomb repressive complex 2 (PRC2) which methylates of histone 3 lysine 27 (H3K27). In this work, we show that JARID2 and the PRC2 complex regulate the cell cycle in skeletal muscle cells to control proliferation and mitotic exit. We found that the stable depletion of JARID2 leads to increased proliferation and cell accumulation in S phase. The regulation of the cell cycle by JARID2 is mediated by direct repression of both cyclin D1 and cyclin E1, both of which are targets of PRC2-mediated H3K27 methylation. Intriguingly, we also find that the retinoblastoma protein (RB1) is a direct target of JARID2 and the PRC2 complex. The depletion of JARID2 is not sufficient to activate RB1. However, the ectopic expression of RB1 can suppress cyclin D1 expression in JARID2-depleted cells. Transient depletion of JARID2 in skeletal muscle cells leads to a transient up-regulation of cyclin D1 that is quickly suppressed with no resulting effect on proliferation, Taken together, we show that JARID2 and the PRC2 complex regulate skeletal muscle proliferation in a precise manner that involves the repression of cyclin D1, thus restraining proliferation and repressing RB1, which is required for mitotic exit and terminal differentiation.
Keywords: EZH2; JARID2; Jumonji; cell cycle; chromatin state; cyclin D1; cyclin E1; gene regulation; histone demethylase; polycomb; polycomb repressive complex 2 (PRC2); retinoblastoma protein (pRb, RB); skeletal muscle.
© 2019 Adhikari et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

