Small-Molecule Antiviral β-d- N4-Hydroxycytidine Inhibits a Proofreading-Intact Coronavirus with a High Genetic Barrier to Resistance
- PMID: 31578288
- PMCID: PMC6880162
- DOI: 10.1128/JVI.01348-19
Small-Molecule Antiviral β-d- N4-Hydroxycytidine Inhibits a Proofreading-Intact Coronavirus with a High Genetic Barrier to Resistance
Abstract
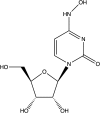
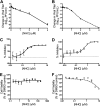
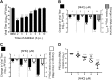
Coronaviruses (CoVs) have emerged from animal reservoirs to cause severe and lethal disease in humans, but there are currently no FDA-approved antivirals to treat the infections. One class of antiviral compounds, nucleoside analogues, mimics naturally occurring nucleosides to inhibit viral replication. While these compounds have been successful therapeutics for several viral infections, mutagenic nucleoside analogues, such as ribavirin and 5-fluorouracil, have been ineffective at inhibiting CoVs. This has been attributed to the proofreading activity of the viral 3'-5' exoribonuclease (ExoN). β-d-N4-Hydroxycytidine (NHC) (EIDD-1931; Emory Institute for Drug Development) has recently been reported to inhibit multiple viruses. Here, we demonstrate that NHC inhibits both murine hepatitis virus (MHV) (50% effective concentration [EC50] = 0.17 μM) and Middle East respiratory syndrome CoV (MERS-CoV) (EC50 = 0.56 μM) with minimal cytotoxicity. NHC inhibited MHV lacking ExoN proofreading activity similarly to wild-type (WT) MHV, suggesting an ability to evade or overcome ExoN activity. NHC inhibited MHV only when added early during infection, decreased viral specific infectivity, and increased the number and proportion of G:A and C:U transition mutations present after a single infection. Low-level NHC resistance was difficult to achieve and was associated with multiple transition mutations across the genome in both MHV and MERS-CoV. These results point to a virus-mutagenic mechanism of NHC inhibition in CoVs and indicate a high genetic barrier to NHC resistance. Together, the data support further development of NHC for treatment of CoVs and suggest a novel mechanism of NHC interaction with the CoV replication complex that may shed light on critical aspects of replication.IMPORTANCE The emergence of coronaviruses (CoVs) into human populations from animal reservoirs has demonstrated their epidemic capability, pandemic potential, and ability to cause severe disease. However, no antivirals have been approved to treat these infections. Here, we demonstrate the potent antiviral activity of a broad-spectrum ribonucleoside analogue, β-d-N4-hydroxycytidine (NHC), against two divergent CoVs. Viral proofreading activity does not markedly impact sensitivity to NHC inhibition, suggesting a novel interaction between a nucleoside analogue inhibitor and the CoV replicase. Further, passage in the presence of NHC generates only low-level resistance, likely due to the accumulation of multiple potentially deleterious transition mutations. Together, these data support a mutagenic mechanism of inhibition by NHC and further support the development of NHC for treatment of CoV infections.
Keywords: MERS-CoV; RNA-dependent RNA polymerase; RdRp; SARS-CoV; antiviral resistance; coronavirus; nucleoside analogue; pandemic.
Copyright © 2019 American Society for Microbiology.
Figures




References
-
- Ksiazek TG, Erdman D, Goldsmith CS, Zaki SR, Peret T, Emery S, Tong S, Urbani C, Comer JA, Lim W, Rollin PE, Dowell SF, Ling A-E, Humphrey CD, Shieh W-J, Guarner J, Paddock CD, Rota P, Fields B, DeRisi J, Yang J-Y, Cox N, Hughes JM, DeLuc JW, Bellini WJ, Anderson LJ. 2003. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med 348:1953–1966. doi: 10.1056/NEJMoa030781. - DOI - PubMed
-
- Gaunt ER, Hardie A, Claas ECJ, Simmonds P, Templeton KE. 2010. Epidemiology and clinical presentations of the four human coronaviruses 229E, HKU1, NL63, and OC43 detected over 3 years using a novel multiplex real-time PCR method. J Clin Microbiol 48:2940–2947. doi: 10.1128/JCM.00636-10. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

