How HIV Nef Proteins Hijack Membrane Traffic To Promote Infection
- PMID: 31578291
- PMCID: PMC6880166
- DOI: 10.1128/JVI.01322-19
How HIV Nef Proteins Hijack Membrane Traffic To Promote Infection
Abstract
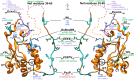
The accessory protein Nef of human immunodeficiency virus (HIV) is a primary determinant of viral pathogenesis. Nef is abundantly expressed during infection and reroutes a variety of cell surface proteins to disrupt host immunity and promote the viral replication cycle. Nef counteracts host defenses by sequestering and/or degrading its targets via the endocytic and secretory pathways. Nef does this by physically engaging a number of host trafficking proteins. Substantial progress has been achieved in identifying the targets of Nef, and a structural and mechanistic understanding of Nef's ability to command the protein trafficking machinery has recently started to coalesce. Comparative analysis of HIV and simian immunodeficiency virus (SIV) Nef proteins in the context of recent structural advances sheds further light on both viral evolution and the mechanisms whereby trafficking is hijacked. This review describes how advances in cell and structural biology are uncovering in growing detail how Nef subverts the host immune system, facilitates virus release, and enhances viral infectivity.
Keywords: Nef; antiviral restriction factors; host-pathogen; human immunodeficiency virus; immune receptors; lentiviruses; protein structure; simian immunodeficiency virus; trafficking.
Copyright © 2019 American Society for Microbiology.
Figures



References
-
- Fackler OT, Kienzle N, Kremmer E, Boese A, Schramm B, Klimkait T, Kucherer C, Mueller-Lantzsch N. 1997. Association of human immunodeficiency virus Nef protein with actin is myristoylation dependent and influences its subcellular localization. Eur J Biochem 247:843–851. doi:10.1111/j.1432-1033.1997.00843.x. - DOI - PubMed

