Trimethoprim and other nonclassical antifolates an excellent template for searching modifications of dihydrofolate reductase enzyme inhibitors
- PMID: 31578455
- PMCID: PMC7102388
- DOI: 10.1038/s41429-019-0240-6
Trimethoprim and other nonclassical antifolates an excellent template for searching modifications of dihydrofolate reductase enzyme inhibitors
Abstract
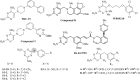
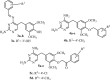
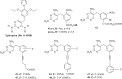
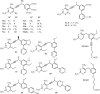
The development of new mechanisms of resistance among pathogens, the occurrence and transmission of genes responsible for antibiotic insensitivity, as well as cancer diseases have been a serious clinical problem around the world for over 50 years. Therefore, intense searching of new leading structures and active substances, which may be used as new drugs, especially against strain resistant to all available therapeutics, is very important. Dihydrofolate reductase (DHFR) has attracted a lot of attention as a molecular target for bacterial resistance over several decades, resulting in a number of useful agents. Trimethoprim (TMP), (2,4-diamino-5-(3',4',5'-trimethoxybenzyl)pyrimidine) is the well-known dihydrofolate reductase inhibitor and one of the standard antibiotics used in urinary tract infections (UTIs). This review highlights advances in design, synthesis, and biological evaluations in structural modifications of TMP as DHFR inhibitors. In addition, this report presents the differences in the active site of human and pathogen DHFR. Moreover, an excellent review of DHFR inhibition and their relevance to antimicrobial and parasitic chemotherapy was presented.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures






References
-
- Schnell JR, Dyson HJ, Wright PE. Structure, dynamics, and catalytic function of dihydrofolate reductase. Annu Rev Biophys Biomol Struct. 2004;33:119–40. - PubMed
-
- Schweitzer B, Dicker AP, Bertino JR. Dihydrofolate reductase as a therapeutic target. FASEB J. 1990;4:2441–52. - PubMed
-
- Blakley RL. Eukaryotic dihydrofolate reductase. Adv Enzymol Relat Areas Mol Biol. 1995;70:23–102. - PubMed
-
- Heaslet H, Harris M, Fahnoe K, et al. Structural comparison of chromosomal and exogenous dihydrofolate reductase from Staphylococcus aureus in complex with the potent inhibitor trimethoprim. Proteins. 2009;76:706–17. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

