A quantitative neuropathological assessment of translocator protein expression in multiple sclerosis
- PMID: 31578541
- PMCID: PMC6821167
- DOI: 10.1093/brain/awz287
A quantitative neuropathological assessment of translocator protein expression in multiple sclerosis
Abstract
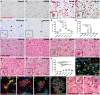
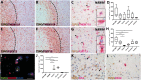
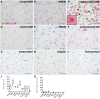
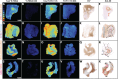
The 18 kDa translocator protein (TSPO) is increasingly used to study brain and spinal cord inflammation in degenerative diseases of the CNS such as multiple sclerosis. The enhanced TSPO PET signal that arises during disease is widely considered to reflect activated pathogenic microglia, although quantitative neuropathological data to support this interpretation have not been available. With the increasing interest in the role of chronic microglial activation in multiple sclerosis, characterising the cellular neuropathology associated with TSPO expression is of clear importance for understanding the cellular and pathological processes on which TSPO PET imaging is reporting. Here we have studied the cellular expression of TSPO and specific binding of two TSPO targeting radioligands (3H-PK11195 and 3H-PBR28) in tissue sections from 42 multiple sclerosis cases and 12 age-matched controls. Markers of homeostatic and reactive microglia, astrocytes, and lymphocytes were used to investigate the phenotypes of cells expressing TSPO. There was an approximate 20-fold increase in cells double positive for TSPO and HLA-DR in active lesions and in the rim of chronic active lesion, relative to normal appearing white matter. TSPO was uniformly expressed across myeloid cells irrespective of their phenotype, rather than being preferentially associated with pro-inflammatory microglia or macrophages. TSPO+ astrocytes were increased up to 7-fold compared to normal-appearing white matter across all lesion subtypes and accounted for 25% of the TSPO+ cells in these lesions. To relate TSPO protein expression to ligand binding, specific binding of the TSPO ligands 3H-PK11195 and 3H-PBR28 was determined in the same lesions. TSPO radioligand binding was increased up to seven times for 3H-PBR28 and up to two times for 3H-PK11195 in active lesions and the centre of chronic active lesions and a strong correlation was found between the radioligand binding signal for both tracers and the number of TSPO+ cells across all of the tissues examined. In summary, in multiple sclerosis, TSPO expression arises from microglia of different phenotypes, rather than being restricted to microglia which express classical pro-inflammatory markers. While the majority of cells expressing TSPO in active lesions or chronic active rims are microglia/macrophages, our findings also emphasize the significant contribution of activated astrocytes, as well as smaller contributions from endothelial cells. These observations establish a quantitative framework for interpretation of TSPO in multiple sclerosis and highlight the need for neuropathological characterization of TSPO expression for the interpretation of TSPO PET in other neurodegenerative disorders.
Keywords: astrocytes; microglia; multiple sclerosis; positron emission tomography; translocator protein.
© The Author(s) (2019). Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures


References
-
- Agnello D, Carvelli L, Muzio V, Villa P, Bottazzi B, Polentarutti N, et al.Increased peripheral benzodiazepine binding sites and pentraxin 3 expression in the spinal cord during EAE: relation to inflammatory cytokines and modulation by dexamethasone and rolipram. J Neuroimmunol 2000; 109: 105–11. - PubMed
-
- Arlicot N, Katsifis A, Garreau L, Mattner F, Vergote J, Duval S, et al.Evaluation of CLINDE as potent translocator protein (18 kDa) SPECT radiotracer reflecting the degree of neuroinflammation in a rat model of microglial activation. Eur J Nucl Med Mol Imaging 2008; 35: 2203–11. - PubMed
-
- Banati RB, Goerres GW, Myers R, Gunn RN, Turkheimer FE, Kreutzberg GW, et al.[11C](R)-PK11195 positron emission tomography imaging of activated microglia in vivo in Rasmussen’s encephalitis. Neurology 1999; 53: 2199. - PubMed
-
- Banati RB, Newcombe J, Gunn RN, Cagnin A, Turkheimer F, Heppner F, et al.The peripheral benzodiazepine binding site in the brain in multiple sclerosis-quantitative in vivo imaging of microglia as a measure of disease activity. Brain 2000; 123: 2321–37. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

