Getting the Right Grip? How Understanding Electrophile Selectivity Profiles Could Illuminate Our Understanding of Redox Signaling
- PMID: 31578876
- PMCID: PMC7583342
- DOI: 10.1089/ars.2019.7894
Getting the Right Grip? How Understanding Electrophile Selectivity Profiles Could Illuminate Our Understanding of Redox Signaling
Abstract
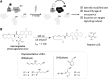
Significance: Electrophile signaling is coming into focus as a bona fide cell signaling mechanism. The electrophilic regulation occurs typically through a sensing event (i.e., labeling of a protein) and a signaling event (the labeling event having an effect of the proteins activity, association, etc.). Recent Advances: Herein, we focus on the first step of this process, electrophile sensing. Electrophile sensing is typically a deceptively simple reaction between the thiol of a protein cysteine, of which there are around 200,000 in the human proteome, and a Michael acceptor, of which there are numerous flavors, including enals and enones. Recent data overall paint a picture that despite being a simple chemical reaction, electrophile sensing is a discerning process, showing labeling preferences that are often not in line with reactivity of the electrophile. Critical Issues: With a view to trying to decide what brings about highly electrophile-reactive protein cysteines, and how reactive these sensors may be, we discuss aspects of the thermodynamics and kinetics of covalent/noncovalent binding. Data made available by several laboratories indicate that it is likely that specific proteins exhibit highly stereo- and chemoselective electrophile sensing, which we take as good evidence for recognition between the electrophile and the protein before forming a covalent bond. Future Directions: We propose experiments that could help us gain a better and more quantitative understanding of the mechanisms through which sensing comes about. We further extoll the importance of performing more detailed experiments on labeling and trying to standardize the way we assess protein-specific electrophile sensing.
Keywords: affinity; covalent labeling; electrophile signaling; kinetic control; mechanism; stereoselectivity.
Figures





Similar articles
-
REX technologies for profiling and decoding the electrophile signaling axes mediated by Rosetta Stone proteins.Methods Enzymol. 2020;633:203-230. doi: 10.1016/bs.mie.2019.02.039. Epub 2019 Mar 14. Methods Enzymol. 2020. PMID: 32046846 Free PMC article.
-
Privileged Electrophile Sensors: A Resource for Covalent Drug Development.Cell Chem Biol. 2017 Jul 20;24(7):787-800. doi: 10.1016/j.chembiol.2017.05.023. Epub 2017 Jun 22. Cell Chem Biol. 2017. PMID: 28648380 Free PMC article. Review.
-
Detection of electrophile-sensitive proteins.Biochim Biophys Acta. 2014 Feb;1840(2):913-22. doi: 10.1016/j.bbagen.2013.09.003. Epub 2013 Sep 8. Biochim Biophys Acta. 2014. PMID: 24021887 Free PMC article. Review.
-
redox Signaling by 8-nitro-cyclic guanosine monophosphate: nitric oxide- and reactive oxygen species-derived electrophilic messenger.Antioxid Redox Signal. 2013 Oct 10;19(11):1236-46. doi: 10.1089/ars.2012.5067. Epub 2013 Jan 3. Antioxid Redox Signal. 2013. PMID: 23157314 Review.
-
An Oculus to Profile and Probe Target Engagement In Vivo: How T-REX Was Born and Its Evolution into G-REX.Acc Chem Res. 2021 Feb 2;54(3):618-631. doi: 10.1021/acs.accounts.0c00537. Epub 2020 Nov 23. Acc Chem Res. 2021. PMID: 33228351
Cited by
-
The mRNA-Binding Protein HuR Is a Kinetically-Privileged Electrophile Sensor.Helv Chim Acta. 2020 May;103(5):e2000041. doi: 10.1002/hlca.202000041. Epub 2020 Apr 12. Helv Chim Acta. 2020. PMID: 34113045 Free PMC article.
-
A primer on harnessing non-enzymatic post-translational modifications for drug design.RSC Med Chem. 2021 Oct 26;12(11):1797-1807. doi: 10.1039/d1md00157d. eCollection 2021 Nov 17. RSC Med Chem. 2021. PMID: 34825181 Free PMC article.
References
-
- Bar-Even A, Noor E, Savir Y, Liebermeister W, Davidi D, Tawfik DS, and Milo R. The moderately efficient enzyme: evolutionary and physicochemical trends shaping enzyme parameters. Biochemistry 50: 4402–4410, 2011 - PubMed
-
- Barlow R. Enantiomers: how valid is Pfeiffer's rule? Trends Pharmacol Sci 11: 148–150, 1990 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

