Multiple threading of a triple-calix[6]arene host
- PMID: 31579081
- PMCID: PMC6753684
- DOI: 10.3762/bjoc.15.207
Multiple threading of a triple-calix[6]arene host
Abstract
The synthesis of the triple-calix[6]arene derivative 6 in which three calix[6]arene macrocycles are linked to a central 1,3,5-trimethylbenzene moiety is reported. Derivative 6 is able to give multiple-threading processes in the presence of dialkylammonium axles. The formation of pseudo[2]rotaxane, pseudo[3]rotaxane, and pseudo[4]rotaxane by threading one, two, and three, respectively, calix-wheels of 6 has been studied by 1D and 2D NMR, DOSY, and ESI-FT-ICR MS/MS experiments. The use of a directional alkylbenzylammonium axle led to the stereoselective formation of endo-alkyl pseudo[n]rotaxane stereoisomers.
Keywords: calixarene; multiple-threading; pseudo[n]rotaxane; stereoisomers.
Copyright © 2019, Iuliano et al.; licensee Beilstein-Institut.
Figures



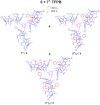
 6, (7+)2
6, (7+)2 6, (7+)3
6, (7+)3 6 pseudorotaxane architectures by multiple-threading of 6 with 7+ as TFPB− salt.
6 pseudorotaxane architectures by multiple-threading of 6 with 7+ as TFPB− salt.
 6. (Top a–c) Significant portions of: (a) 1H NMR spectrum of 6 (CDCl3, 298 K, 600 MHz); (b) 1H NMR spectrum of a 1:1 mixture of 6 and 7+·TFPB− (CDCl3, 298 K, 600 MHz); (c) DOSY spectra of 6 (black line) and a 1:1 mixture of 6 and 7+·TFPB− (red line). Inset: structures of the triple-calix[6]arene 6 and 7+
6. (Top a–c) Significant portions of: (a) 1H NMR spectrum of 6 (CDCl3, 298 K, 600 MHz); (b) 1H NMR spectrum of a 1:1 mixture of 6 and 7+·TFPB− (CDCl3, 298 K, 600 MHz); (c) DOSY spectra of 6 (black line) and a 1:1 mixture of 6 and 7+·TFPB− (red line). Inset: structures of the triple-calix[6]arene 6 and 7+ 6 pseudo[2]rotaxane obtained by molecular mechanics calculations.
6 pseudo[2]rotaxane obtained by molecular mechanics calculations.
 6; purple circle = (7+)2
6; purple circle = (7+)2 6; green triangle = (7+)3
6; green triangle = (7+)3 6.
6.
 6.
6.
 6 obtained by molecular mechanics calculations.
6 obtained by molecular mechanics calculations.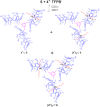
 6, (4+)2
6, (4+)2 6, (4+)3
6, (4+)3 6 pseudorotaxane architectures by multiple-threading of 6 with 4+ as TFPB− salt.
6 pseudorotaxane architectures by multiple-threading of 6 with 4+ as TFPB− salt.
 6. (f) HR-ESI-FT-ICR-CID mass spectrum of (4+)2
6. (f) HR-ESI-FT-ICR-CID mass spectrum of (4+)2 6.
6.
 6 obtained by molecular mechanics calculations.
6 obtained by molecular mechanics calculations.

 6. (Right) HR-ESI-FT-ICR-CID mass spectrum of (8+)2
6. (Right) HR-ESI-FT-ICR-CID mass spectrum of (8+)2 6.
6.
 6, (8+)2
6, (8+)2 6, (8+)3
6, (8+)3 6 pseudorotaxane architectures (sketch) by multiple-threading of 6 with 8+ as TFPB− salt.
6 pseudorotaxane architectures (sketch) by multiple-threading of 6 with 8+ as TFPB− salt.References
-
- Swiegers G F. Self-Assembly: Terminology. In: Steed J W, Atwood J L, editors. Encylopedia of Supramolecular Chemistry. New York, NY, USA: Marcel Dekker; 2004. pp. 1263–1269. - DOI
-
- Yao X, Ma X, Tian H. J Mater Chem C. 2019 doi: 10.1039/c9tc03257f. - DOI
-
- Bruns C J, Stoddart J F. The Nature of the Mechanical Bond: From Molecules to Machines. 1st ed. Hoboken, New Jersey: John Wiley & Sons; 2017. - DOI
LinkOut - more resources
Full Text Sources
