Ginsenoside Rh1 inhibits colorectal cancer cell migration and invasion in vitro and tumor growth in vivo
- PMID: 31579419
- PMCID: PMC6757309
- DOI: 10.3892/ol.2019.10742
Ginsenoside Rh1 inhibits colorectal cancer cell migration and invasion in vitro and tumor growth in vivo
Abstract
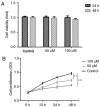
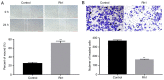
Colorectal cancer (CRC) is the third leading cause of cancer-associated mortality worldwide. Ginsenoside Rh1 (Rh1) is a traditional medicine monomer with antitumor activity; however, the effects of Rh1 in CRC remain to be determined. In the present study, SW620 cells were treated with different concentrations of Rh1. Cell Counting Kit-8, wound healing and Transwell assays were performed to measure cell viability and proliferation, migration and invasion, respectively. Subsequently, the mRNA expression levels of matrix metallopeptidase (MMP)1, MMP3 and tissue inhibitor of metalloproteinases 3 (TIMP3) were detected by reverse transcription-quantitative PCR analysis. In addition, the protein expression levels of MMP1, MMP3, TIMP3, and total or phosphorylated (p-)ERK1/2, P38, JNK were detected by western blotting. Furthermore, tumor growth was examined in a nude mouse xenograft model. The results of the present study indicated that Rh1 was not toxic to CRC cells at various concentrations (0, 50 or 100 µM) and treatment durations (24 or 48 h). However, cell proliferation was suppressed by Rh1 in a dose-dependent manner. Rh1 (100 µM) significantly inhibited cell migration and invasion in vitro. Additionally, Rh1 suppressed the mRNA and protein expression of MMP1 and MMP3, and promoted TIMP3 expression. Rh1 decreased the ratios of p-P38/P38, p-ERK1/2/ERK1-2 and p-JNK/JNK in vitro and in vivo, which suggested that Rh1 inactivated the mitogen-activated protein kinase (MAPK) signaling pathway. Notably, Rh1 markedly decreased tumor volume and weight in vivo. In conclusion, the present study demonstrated that Rh1 inhibited the proliferation, migration and invasion of CRC cells in vitro and tumor growth in vivo. This inhibition was at least partially due to the inhibition of MMP1 and MMP3 expression, the increase in TIMP3 expression level and the MAPK signaling pathway inactivation. Therefore, Rh1 may effectively inhibit the development of CRC as an anticancer drug, and may have a supporting effect during CRC treatment.
Keywords: colorectal cancer; ginsenoside Rh1; invasion; migration; tumor growth.
Copyright: © Lyu et al.
Figures


Similar articles
-
Ginsenoside Rh1 suppresses matrix metalloproteinase-1 expression through inhibition of activator protein-1 and mitogen-activated protein kinase signaling pathway in human hepatocellular carcinoma cells.Eur J Pharmacol. 2012 Mar 15;679(1-3):24-33. doi: 10.1016/j.ejphar.2012.01.020. Epub 2012 Jan 31. Eur J Pharmacol. 2012. PMID: 22314224
-
Protopanaxatriol ginsenoside Rh1 inhibits the expression of matrix metalloproteinases and the in vitro invasion/migration of human astroglioma cells.Neurochem Int. 2013 Aug;63(2):80-6. doi: 10.1016/j.neuint.2013.05.002. Epub 2013 May 15. Neurochem Int. 2013. PMID: 23684955
-
Sea cucumber extract TBL-12 inhibits the proliferation, migration, and invasion of human prostate cancer cells through the p38 mitogen-activated protein kinase and intrinsic caspase apoptosis pathway.Prostate. 2019 Jun;79(8):826-839. doi: 10.1002/pros.23788. Epub 2019 Mar 19. Prostate. 2019. PMID: 30889629
-
Ginsenoside Rh1 regulates gastric cancer cell biological behaviours and transplanted tumour growth in nude mice via the TGF-β/Smad pathway.Clin Exp Pharmacol Physiol. 2022 Dec;49(12):1270-1280. doi: 10.1111/1440-1681.13708. Epub 2022 Aug 30. Clin Exp Pharmacol Physiol. 2022. PMID: 36054718
-
Ginsenoside Rh1 Inhibits Angiotensin II-Induced Vascular Smooth Muscle Cell Migration and Proliferation through Suppression of the ROS-Mediated ERK1/2/p90RSK/KLF4 Signaling Pathway.Antioxidants (Basel). 2022 Mar 27;11(4):643. doi: 10.3390/antiox11040643. Antioxidants (Basel). 2022. PMID: 35453328 Free PMC article.
Cited by
-
Proteomic Analyses of Fibroblast- and Serum-Derived Exosomes Identify QSOX1 as a Marker for Non-invasive Detection of Colorectal Cancer.Cancers (Basel). 2021 Mar 17;13(6):1351. doi: 10.3390/cancers13061351. Cancers (Basel). 2021. PMID: 33802764 Free PMC article.
-
Traditional Chinese medicine for colorectal cancer treatment: potential targets and mechanisms of action.Chin Med. 2023 Feb 13;18(1):14. doi: 10.1186/s13020-023-00719-7. Chin Med. 2023. PMID: 36782251 Free PMC article. Review.
-
Anti-Metastatic and Anti-Inflammatory Effects of Matrix Metalloproteinase Inhibition by Ginsenosides.Biomedicines. 2021 Feb 17;9(2):198. doi: 10.3390/biomedicines9020198. Biomedicines. 2021. PMID: 33671187 Free PMC article. Review.
-
Ginsenoside Rh1 inhibits tumor growth in MDA-MB-231 breast cancer cells via mitochondrial ROS and ER stress-mediated signaling pathway.Arch Pharm Res. 2022 Mar;45(3):174-184. doi: 10.1007/s12272-022-01377-3. Epub 2022 Mar 24. Arch Pharm Res. 2022. PMID: 35325393
-
Investigating the Anticancer Activity of G-Rh1 Using In Silico and In Vitro Studies (A549 Lung Cancer Cells).Molecules. 2022 Nov 28;27(23):8311. doi: 10.3390/molecules27238311. Molecules. 2022. PMID: 36500403 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
