Adverse events associated with home use of mouthrinses: a systematic review
- PMID: 31579502
- PMCID: PMC6759706
- DOI: 10.1177/2042098619854881
Adverse events associated with home use of mouthrinses: a systematic review
Abstract
Background: Poor oral hygiene is strongly associated with oral and systemic diseases. Alongside mechanical tooth cleaning, the adjunctive use of mouthrinses has been widely advocated. Although research on the efficacy of various mouthrinse formulations is very active, there are a lack of conclusive data regarding their adverse effects.
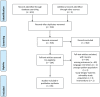
Methods: We undertook a systematic review in accordance wih PRISMA guidelines of electronic databases of clinical trials of any duration with daily home use of mouthwashes, presenting clinical and subjective side effects (PROSPERO registration: CRD42016054037).
Results: After evaluating 614 titles and abstracts, 154 studies were selected for full-text analysis; 85 final papers were included. Based on the active ingredient in the test product, nine categories were created: cetyl pyridinium chloride, essential oils, chlorhexidine, triclosan, natural products, diclofenac, fluorides, delmopinol, and miscellaneous active substances. Most of the studies were of short duration (less than 6 months) with a defective 'methods' description; the reporting of adverse events often being overlooked. Both local morphological (oral mucosa and dental-crown staining, mucosal lesions) and functional (taste modifications, abnormal oral sensation) alterations were reported. Tooth staining was the most commonly listed adverse event, but it was quantitatively assessed only in a very small number of papers; most studies relied on patient reports. Staining was time associated; the longer the study, the higher its reported incidence and severity.
Conclusions: The reduced report of side effects may partly be due to a lack of an objective measure and lack of general guidelines that demand studies report their adverse events. The most frequently reported adverse effect was teeth staining. As in most studies, the effect was associated with trial duration; clinical trials should be of sufficient duration. New investigations meeting the suggested criteria of a minimal duration of 6 months should be planned.
Keywords: adverse effects; chemical compounds; mouthrinse; oral health; oral staining.
© The Author(s), 2019.
Conflict of interest statement
Conflict of interest statement: The authors declare that there is no conflict of interest.
Figures
References
-
- Gurenlian JR. The role of dental plaque biofilm in oral health. J Dent Hyg 2007; 81: 1–11.
-
- Loe H. Oral hygiene in the prevention of caries and periodontal disease. Int Dent J 2000; 50: 129–139. - PubMed
-
- Shahid M. Regular supervised fluoride mouthrinse use by children and adolescents associated with caries reduction. Evid Based Dent 2017; 18: 11–12. - PubMed
-
- Tartaglia GM, Kumar S, Fornari CD, et al. Mouthwashes in the 21st century: a narrative review about active molecules and effectiveness on the periodontal outcomes. Expert Opin Drug Deliv 2017; 14: 973–982. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Medical
Research Materials