Comparison of bleeding risks among non-vitamin K antagonist oral anticoagulants using the Korea adverse event reporting system database
- PMID: 31579503
- PMCID: PMC6759695
- DOI: 10.1177/2042098619876737
Comparison of bleeding risks among non-vitamin K antagonist oral anticoagulants using the Korea adverse event reporting system database
Abstract
Background: In order to ensure safer use of non-vitamin K antagonist oral anticoagulants (NOACs), continuously detecting unexpected adverse drug reactions (ADRs) after market approval is necessary.
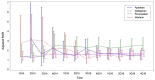
Methods: We performed disproportionality analysis to evaluate association between ADRs and NOACs including apixaban, dabigatran, and rivaroxaban using data from the Korea Institute of Drug Safety and Risk Management-Korea Adverse Event Reporting System database (KIDS-KD) between 2012 and 2016. There was no significant signal other than bleeding when considering quantity, signal strength, seriousness, and causality. In order to evaluate the NOAC reports about bleeding, we selected 62 WHO-ART diagnostic codes associated with bleeding. Among the 26 codes that referred to major bleeding, 18 codes referred to gastrointestinal bleeding and 8 were referred to intracranial bleeding. We evaluated the significance of the signals using reporting odds ratios (RORs) adjusted for age and sex.
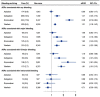
Results: Treatments with apixaban, dabigatran, and rivaroxaban were associated with 1989, 1668, and 2960 adverse events, respectively. Any type of bleeding with apixaban, dabigatran, rivaroxaban, and warfarin was reported in 174 (8.8%), 209 (12.5%), 523 (17.8%), and 620 (9.5%) events, respectively. For any bleeding, adjusted RORs of apixaban, dabigatran, and rivaroxaban were 0.99 [95% confidence interval (CI): 0.83-1.17], 1.47 (95% CI: 1.25-1.75), and 2.48 (95% CI: 2.16-2.84), respectively. With respect to major bleeding, the adjusted RORs of apixaban, dabigatran, and rivaroxaban were 1.08 (95% CI: 0.82-1.41), 1.46 (95% CI: 1.10-1.90), and 1.82 (95% CI: 1.43-2.32), respectively.
Conclusion: Rivaroxaban might have stronger association with bleeding than apixaban and dabigatran.
Keywords: adverse events; bleeding; non-vitamin K antagonist oral anticoagulants; oral anticoagulants.
© The Author(s), 2019.
Conflict of interest statement
Conflict of interest statement: The authors declare that there is no conflict of interest.
Figures


Similar articles
-
Comparative Stroke, Bleeding, and Mortality Risks in Older Medicare Patients Treated with Oral Anticoagulants for Nonvalvular Atrial Fibrillation.Am J Med. 2019 May;132(5):596-604.e11. doi: 10.1016/j.amjmed.2018.12.023. Epub 2019 Jan 9. Am J Med. 2019. PMID: 30639551
-
Effectiveness and Safety of Contemporary Oral Anticoagulants Among Asians With Nonvalvular Atrial Fibrillation.Stroke. 2019 Aug;50(8):2245-2249. doi: 10.1161/STROKEAHA.119.025536. Epub 2019 Jun 18. Stroke. 2019. PMID: 31208303
-
Evaluation of rivaroxaban-, apixaban- and dabigatran-associated hemorrhagic events using the FDA-Adverse event reporting system (FAERS) database.Int J Clin Pharm. 2021 Dec;43(6):1508-1515. doi: 10.1007/s11096-021-01273-8. Epub 2021 Jun 9. Int J Clin Pharm. 2021. PMID: 34109494
-
Association of Intracranial Hemorrhage Risk With Non-Vitamin K Antagonist Oral Anticoagulant Use vs Aspirin Use: A Systematic Review and Meta-analysis.JAMA Neurol. 2018 Dec 1;75(12):1511-1518. doi: 10.1001/jamaneurol.2018.2215. JAMA Neurol. 2018. PMID: 30105396 Free PMC article.
-
Direct comparative effectiveness and safety between non-vitamin K antagonist oral anticoagulants for stroke prevention in nonvalvular atrial fibrillation: a systematic review and meta-analysis of observational studies.Eur J Epidemiol. 2019 Feb;34(2):173-190. doi: 10.1007/s10654-018-0415-7. Epub 2018 Jun 8. Eur J Epidemiol. 2019. PMID: 29948370
Cited by
-
Real-Time Imaging of Platelet-Initiated Plasma Clot Formation and Lysis Unveils Distinct Impacts of Anticoagulants.Thromb Haemost. 2025 Aug;125(8):766-778. doi: 10.1055/a-2497-4213. Epub 2025 Jan 9. Thromb Haemost. 2025. PMID: 39788528 Free PMC article.
-
The Impact of Novel Anticoagulants on the Upper Gastrointestinal Tract Mucosa.Medicina (Kaunas). 2020 Jul 21;56(7):363. doi: 10.3390/medicina56070363. Medicina (Kaunas). 2020. PMID: 32708292 Free PMC article.
-
Causality assessment between reported fatal cerebral haemorrhage and suspected drugs: developing a new algorithm based on the analysis of the Japanese Adverse Event Report (JADER) database and literature review.Eur J Clin Pharmacol. 2021 Oct;77(10):1443-1452. doi: 10.1007/s00228-021-03131-y. Epub 2021 Apr 7. Eur J Clin Pharmacol. 2021. PMID: 33829295 Review.
-
Comparative effectiveness and safety of non-vitamin K antagonists for atrial fibrillation in clinical practice: GLORIA-AF Registry.Clin Res Cardiol. 2022 May;111(5):560-573. doi: 10.1007/s00392-022-01996-2. Epub 2022 Mar 16. Clin Res Cardiol. 2022. PMID: 35294625 Free PMC article.
-
Analysis of Adverse Events in the Treatment of Patients with Non-Valvular Atrial Fibrillation with Oral Anticoagulants: Data from the "ANTEY" Observational Study.Pharmaceuticals (Basel). 2022 Sep 29;15(10):1209. doi: 10.3390/ph15101209. Pharmaceuticals (Basel). 2022. PMID: 36297321 Free PMC article.
References
-
- Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 1991; 22: 983–988. - PubMed
-
- Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet 2014; 383: 955–962. - PubMed
-
- January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation 2014; 130: e199–e267. - PMC - PubMed
-
- Camm AJ, Lip GY, De Caterina R, et al. 2012 focused update of the ESC guidelines for the management of atrial fibrillation: an update of the 2010 ESC guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J 2012; 33: 2719–2747. - PubMed
LinkOut - more resources
Full Text Sources

