Treatment of pulmonary thromboembolism with edoxaban in a cancer patient with borderline fulfillment of the dose reduction criteria
- PMID: 31579511
- PMCID: PMC6757493
- DOI: 10.1177/2050313X19878047
Treatment of pulmonary thromboembolism with edoxaban in a cancer patient with borderline fulfillment of the dose reduction criteria
Abstract
Venous thromboembolism is the most common cause of death in cancer patients with venous thrombosis. Treatment of venous thrombosis is important in cancer patients, as it can have a major impact on prognosis. We report a case of advanced gastric cancer that was discovered owing to pulmonary thromboembolism and describe the treatment for both conditions. Dose reduction criteria of edoxaban are established. Appropriate dose was based on body weight and creatinine clearance; patients with creatinine clearance values slightly exceeding or below 50 are considered to be on the borderline of the dose reduction criteria. This case had borderline value (body weight: 63 kg, creatinine clearance: 46 mL/min). We observed no response after initiating treatment with 30 mg edoxaban; however, pulmonary thrombus disappeared after increasing the dose to 60 mg edoxaban. When selecting an anticoagulation drug in borderline patients with cancer-associated thrombosis, dose increase should be considered if hemorrhage risk is assessed.
Keywords: Pulmonary thromboembolism; cancer patient; direct oral anticoagulants; edoxaban.
© The Author(s) 2019.
Conflict of interest statement
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures
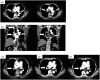
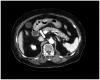
References
-
- Blom JW, Doggen CJ, Osanto S, et al. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA 2005; 293: 715–722. - PubMed
-
- Khorana AA, Francis CW, Culakova E, et al. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J Thromb Haemost 2007; 5(3): 632–634. - PubMed
-
- Sørensen HT, Mellemkjaer L, Olsen JH, et al. Prognosis of cancers associated with venous thromboembolism. N Engl J Med 2000; 343(25): 1846–1850. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

