Induced-Membrane Technique in the Management of Posttraumatic Bone Defects
- PMID: 31579540
- PMCID: PMC6687485
- DOI: 10.2106/JBJS.ST.18.00099
Induced-Membrane Technique in the Management of Posttraumatic Bone Defects
Erratum in
-
Erratum: Induced-Membrane Technique in the Management of Posttraumatic Bone Defects.JBJS Essent Surg Tech. 2020 Jun 2;10(2):e0099ER. doi: 10.2106/JBJS.ST.ER.18.00099. eCollection 2020 Apr-Jun. JBJS Essent Surg Tech. 2020. PMID: 32944416 Free PMC article.
Abstract
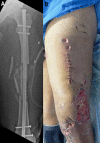
Background: Critical-size bone defects are defined as bone defects where spontaneous regeneration is not expected without treatment1. The characteristics of bone defects (etiology, location, size, presence of infection, and soft-tissue conditions) vary greatly and, to be effective, the treatment method should address this variability. The induced-membrane technique, or Masquelet technique, is a method for treating critical-size bone defects2,3 of various sizes and anatomic locations. It has been used to treat infected and noninfected bone defects and may be performed with a variety of fixation methods2,3.
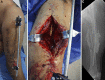
Description: The induced-membrane technique is a 2-stage procedure. The first stage consists of debridement followed by insertion of a polymethylmethacrylate (PMMA) spacer in the bone defect. The presence of the PMMA leads to a foreign-body reaction with the development of a thick pseudosynovial membrane that is extremely vascularized and rich in growth factors. The filling of the bone defect with the cement spacer prevents fibrous tissue invasion and allows the development of an optimal vascularized gap for bone-grafting. After 6 to 8 weeks, the membrane around the spacer is carefully opened for the removal of the spacer, which is then replaced by bone graft2,3, which can be expanded with allograft or biomaterials.
Alternatives: Alternatives include vascularized or nonvascularized autologous bone graft, allograft, bone transport methods, titanium cages, megaprostheses, shortening, and amputation.
Rationale: Posttraumatic bone defects frequently are associated with soft-tissue injury and infection that impair the local vascularization and the healing potential. The highly vascularized induced membrane may play a role in restoring the local regenerative capacity. Numerous studies have demonstrated its successful use in the treatment of posttraumatic bone defects in the hand, forearm, humerus, femur, tibia, and foot. The induced-membrane technique is especially advantageous in the treatment of infected bone defects because the presence of the spacer helps in the treatment of the infection by reducing dead space, acting as a local antibiotic carrier, and promoting some degree of bone stability3-5.
Copyright © 2019 by The Journal of Bone and Joint Surgery, Incorporated.
Figures







References
-
- Schemitsch EH. Size matters: defining critical in bone defect size! J Orthop Trauma. 2017. October;31(Suppl 5):S20-2. - PubMed
-
- Masquelet AC. Induced membrane technique: pearls and pitfalls. J Orthop Trauma. 2017. October;31(Suppl 5):S36-8. - PubMed
-
- Mauffrey C, Hake ME, Chadayammuri V, Masquelet AC. Reconstruction of long bone infections using the induced membrane technique: tips and tricks. J Orthop Trauma. 2016. June;30(6):e188-93. - PubMed
-
- Morelli I, Drago L, George DA, Gallazzi E, Scarponi S, Romanò CL. Masquelet technique: myth or reality? A systematic review and meta-analysis. Injury. 2016. December;47(Suppl 6):S68-76. - PubMed
-
- Azi ML, Teixeira AA, Cotias RB, Joeris A, Kfuri M., Jr Membrane induced osteogenesis in the management of posttraumatic bone defects. J Orthop Trauma. 2016. October;30(10):545-50. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
