Microbial communities associated with the black morel Morchella sextelata cultivated in greenhouses
- PMID: 31579614
- PMCID: PMC6766373
- DOI: 10.7717/peerj.7744
Microbial communities associated with the black morel Morchella sextelata cultivated in greenhouses
Abstract
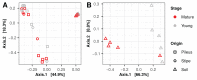
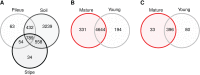
Morels (Morchella spp.) are iconic edible mushrooms with a long history of human consumption. Some microbial taxa are hypothesized to be important in triggering the formation of morel primordia and development of fruiting bodies, thus, there is interest in the microbial ecology of these fungi. To identify and compare fungal and prokaryotic communities in soils where Morchella sextelata is cultivated in outdoor greenhouses, ITS and 16S rDNA high throughput amplicon sequencing and microbiome analyses were performed. Pedobacter, Pseudomonas, Stenotrophomonas, and Flavobacterium were found to comprise the core microbiome of M. sextelata ascocarps. These bacterial taxa were also abundant in the soil beneath growing fruiting bodies. A total of 29 bacterial taxa were found to be statistically associated to Morchella fruiting bodies. Bacterial community network analysis revealed high modularity with some 16S rDNA operational taxonomic unit clusters living in specialized fungal niches (e.g., pileus, stipe). Other fungi dominating the soil mycobiome beneath morels included Morchella, Phialophora, and Mortierella. This research informs understanding of microbial indicators and potential facilitators of Morchella ecology and fruiting body production.
Keywords: Amplicon sequencing; CONSTAX; Microbial ecology; Microbiome; Morchella; Mushroom cultivation; Pedobacter; USEARCH.
© 2019 Benucci et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- Antony-Babu S, Deveau A, Van Nostrand JD, Zhou J, Le Tacon F, Robin C, Frey-Klett P, Uroz S. Black truffle-associated bacterial communities during the development and maturation of Tuber melanosporum ascocarps and putative functional roles. Environmental Microbiology. 2014;16(9):2831–2847. doi: 10.1111/1462-2920.12294. - DOI - PubMed
-
- Aslani MA, Harighi B, Abdollahzadeh J. Screening of endofungal bacteria isolated from wild growing mushrooms as potential biological control agents against brown blotch and internal stipe necrosis diseases of Agaricus bisporus. Biological Control. 2018;119:20–26. doi: 10.1016/j.biocontrol.2018.01.006. - DOI
-
- Barbieri E, Guidi C, Bertaux J, Frey-Klett P, Garbaye J, Ceccaroli P, Saltarelli R, Zambonelli A, Stocchi V. Occurrence and diversity of bacterial communities in Tuber magnatum during truffle maturation. Environmental Microbiology. 2007;9(9):2234–2246. doi: 10.1111/j.1462-2920.2007.01338.x. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

