Corticosteroid tapering with benralizumab treatment for eosinophilic asthma: PONENTE Trial
- PMID: 31579676
- PMCID: PMC6759576
- DOI: 10.1183/23120541.00009-2019
Corticosteroid tapering with benralizumab treatment for eosinophilic asthma: PONENTE Trial
Abstract
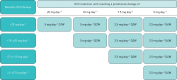
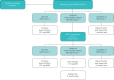
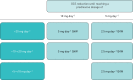
Benralizumab is an interleukin-5 receptor α-directed cytolytic monoclonal antibody approved in several countries for the add-on maintenance treatment of patients with severe eosinophilic asthma aged 12 years and older. In the 28-week Phase III ZONDA trial (ClinicalTrials.gov identifier: NCT02075255), benralizumab produced a median 75% reduction from baseline in oral corticosteroid (OCS) dosage (versus 25% for placebo) while maintaining asthma control for patients with OCS-dependent severe asthma. This manuscript presents the detailed protocol for the Phase IIIb PONENTE (ClinicalTrials.gov identifier: NCT03557307), a study that will build on the findings from ZONDA. As the largest steroid-sparing study undertaken in severe asthma, PONENTE has a faster steroid tapering schedule for prednisone dosages ≥7.5 mg·day-1 than previous studies, and it includes an evaluation of adrenal insufficiency and an algorithm to taper OCS dosage when prednisone dosage is ≤5 mg·day-1. It also has a longer maintenance phase to assess asthma control for up to 6 months after completion of OCS tapering. The two primary endpoints are whether patients achieve 100% reduction in daily OCS use and whether patients achieve 100% reduction in daily OCS or achieve OCS dosage ≤5 mg·day-1, if adrenal insufficiency prevented further reduction, both sustained over ≥4 weeks without worsening of asthma. Safety and change from baseline in health-related quality of life will also be assessed. PONENTE should provide valuable guidance for clinicians on tapering OCS dosage, including the management of adrenal insufficiency, following benralizumab initiation for the treatment of patients who are OCS-dependent with severe, uncontrolled eosinophilic asthma.
Copyright ©ERS 2019.
Conflict of interest statement
Conflict of interest: A. Menzies-Gow reports consultancy agreements with and advisory board membership for AstraZeneca, consultancy agreements with Sanofi and Vectura, advisory board membership for Boehringer Ingelheim, GlaxoSmithKline, Novartis, Sanofi and Teva, receiving speaker fees from AstraZeneca, Boehringer Ingelheim, Novartis, Teva and Vectura, clinical funding from AstraZeneca, having participated in research for which his institution was renumerated by AstraZeneca, and having attended international conferences with sponsorship from Teva and Boehringer Ingelheim, outside the submitted work. Conflict of interest: J. Corren has nothing to disclose. Conflict of interest: E.H. Bel reports grants for research and personal fees from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Novartis, Sanofi-Regeneron, Sterna, Teva, and Vectura. Conflict of interest: J. Maspero reports consultancy for Sanofi, Teva, Novartis and GlaxoSmithKline, speaker fees from Boehringer Ingelheim, AstraZeneca, Menarini, Novartis and Uriach, and research and grants from Sanofi, AstraZeneca and Novartis, outside the submitted work. Conflict of interest: L.G. Heaney has nothing to disclose. Conflict of interest: M. Gurnell has nothing to disclose. Conflict of interest: P. Wessman is an employee of AstraZeneca. Conflict of interest: U.J. Martin is an employee of AstraZeneca. Conflict of interest: S. Siddiqui is an employee of AstraZeneca. Conflict of interest: E. Garcia Gil is an employee of AstraZeneca.
Figures

References
-
- Global Asthma Network. The global asthma report www.globalasthmareport.org Date last accessed: November 21, 2018. Date last updated: 2018.
-
- Global Initiative for Asthma Management and Prevention (GINA). Global strategy for asthma management and prevention www.ginasthma.org/2018-gina-report-global-strategy-for-asthma-management... Date last accessed: November 12, 2018. Date last updated: 2018.
-
- Chung KF, Wenzel SE, Brozek JL, et al. . International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J 2014; 43: 343–373. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
