Complete response after chemoradiotherapy for rectal cancer: what is the reasonable approach?
- PMID: 31579765
- PMCID: PMC6754042
- DOI: 10.1515/iss-2017-0041
Complete response after chemoradiotherapy for rectal cancer: what is the reasonable approach?
Abstract
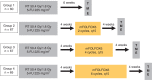
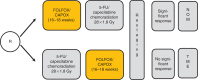
With the increasing use of preoperative treatment rather than upfront surgery, it has become evident that the response of rectal carcinoma to standard chemoradiotherapy (CRT) shows a great variety that includes histopathologiocally confirmed complete tumor regression in 10-30% of cases. Adaptive strategies to avoid radical surgery, either by local excision or non-operative management, have been proposed in these highly responsive tumors. A growing number of prospective clinical trials and experiences from large databases, such as the European Registration of Cancer Care (EURECCA) watch-and-wait database, or the recent Oncological Outcome after Clinical Complete Response in Patients with Rectal Cancer (OnCoRe) project, will provide more information on its safety and efficacy, and help to select appropriate patients. Future studies will have to establish appropriate inclusion criteria and optimize CRT regimens in order to maximize the number of patients achieving complete response. Standardized re-staging procedures have to be investigated to improve the prediction of a sustained complete response, and long-term close follow-up with thorough documentation of failure patterns and salvage therapies will have to prove the oncological safety of this approach.
Keywords: chemoradiotherapy; complete response; local excision; non-operative management; rectal cancer; wait-and-see strategies.
©2018 Rödel C. et al., published by De Gruyter, Berlin/Boston.
Figures
References
-
- Sauer R, Liersch T, Merkel S, Fietkau R, Hohenberger W, Hess C, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow up of 11 years. J Clin Oncol 2012;30:1926–33. - PubMed
- Sauer R, Liersch T, Merkel S, Fietkau R, Hohenberger W, Hess C. et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow up of 11 years. J Clin Oncol. 2012;30:1926–33. - PubMed
-
- van Gijn W, Marijnen CA, Nagtegaal ID, Kranenbarg EM-K, Putter H, Wiggers T, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol 2011;12:575–82. - PubMed
- van Gijn W, Marijnen CA, Nagtegaal ID, Kranenbarg EM-K, Putter H, Wiggers T. et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol. 2011;12:575–82. - PubMed
-
- Fokas E, Liersch T, Fietkau R, Hohenberger W, Beissbarth T, Hess C, et al. Tumor regression grading after preoperative chemoradiotherapy for locally advanced rectal carcinoma revisited: updated results of the CAO/ARO/AIO-94 trial after a median follow-up of 11 years. J Clin Oncol 2014;32:1554–62. - PubMed
- Fokas E, Liersch T, Fietkau R, Hohenberger W, Beissbarth T, Hess C. et al. Tumor regression grading after preoperative chemoradiotherapy for locally advanced rectal carcinoma revisited: updated results of the CAO/ARO/AIO-94 trial after a median follow-up of 11 years. J Clin Oncol. 2014;32:1554–62. - PubMed
-
- Borschitz T, Wachtlin D, Möhler M, Schmidberger H, Junginger T. Neoadjuvant chemoradiation and local excision for T2-3 rectal cancer. Ann Surg Oncol 2008;15:712–20. - PubMed
- Borschitz T, Wachtlin D, Möhler M, Schmidberger H, Junginger T. Neoadjuvant chemoradiation and local excision for T2-3 rectal cancer. Ann Surg Oncol. 2008;15:712–20. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
