Ruminococcin C, a promising antibiotic produced by a human gut symbiont
- PMID: 31579822
- PMCID: PMC6760926
- DOI: 10.1126/sciadv.aaw9969
Ruminococcin C, a promising antibiotic produced by a human gut symbiont
Abstract
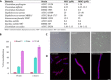
A major public health challenge today is the resurgence of microbial infections caused by multidrug-resistant strains. Consequently, novel antimicrobial molecules are actively sought for development. In this context, the human gut microbiome is an under-explored potential trove of valuable natural molecules, such as the ribosomally-synthesized and post-translationally modified peptides (RiPPs). The biological activity of the sactipeptide subclass of RiPPs remains under-characterized. Here, we characterize an antimicrobial sactipeptide, Ruminococcin C1, purified from the caecal contents of rats mono-associated with Ruminococcus gnavus E1, a human symbiont. Its heterologous expression and post-translational maturation involving a specific sactisynthase establish a thioether network, which creates a double-hairpin folding. This original structure confers activity against pathogenic Clostridia and multidrug-resistant strains but no toxicity towards eukaryotic cells. Therefore, the Ruminococcin C1 should be considered as a valuable candidate for drug development and its producer strain R. gnavus E1 as a relevant probiotic for gut health enhancement.
Copyright © 2019 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
Figures




References
-
- O’Neill A. J., New antibacterial agents for treating infections caused by multi-drug resistant Gram-negative bacteria. Expert Opin. Investig. Drugs 17, 297–302 (2008). - PubMed
-
- J. O’Neill, Tackling Drug-Resistant Infections Globally: Final Report and Recommendations (Review on Antimicrobial Resistance, 2016).
-
- Arnison P. G., Bibb M. J., Bierbaum G., Bowers A. A., Bugni T. S., Bulaj G., Camarero J. A., Campopiano D. J., Challis G. L., Clardy J., Cotter P. D., Craik D. J., Dawson M., Dittmann E., Donadio S., Dorrestein P. C., Entian K.-D., Fischbach M. A., Garavelli J. S., Göransson U., Gruber C. W., Haft D. H., Hemscheidt T. K., Hertweck C., Hill C., Horswill A. R., Jaspars M., Kelly W. L., Klinman J. P., Kuipers O. P., Link A. J., Liu W., Marahiel M. A., Mitchell D. A., Moll G. N., Moore B. S., Müller R., Nair S. K., Nes I. F., Norris G. E., Olivera B. M., Onaka H., Patchett M. L., Piel J., Reaney M. J. T., Rebuffat S., Ross R. P., Sahl H.-G., Schmidt E. W., Selsted M. E., Severinov K., Shen B., Sivonen K., Smith L., Stein T., Süssmuth R. D., Tagg J. R., Tang G.-L., Truman A. W., Vederas J. C., Walsh C. T., Walton J. D., Wenzel S. C., Willey J. M., van der Donk W. A., Ribosomally synthesized and post-translationally modified peptide natural products: Overview and recommendations for a universal nomenclature. Nat. Prod. Rep. 30, 108–160 (2013). - PMC - PubMed
-
- M. C. Rea, R. P. Ross, P. D. Cotter, C. Hill, Classification of bacterocins from Gram-positive bacteria, in Prokaryotic Antimicrobial Peptides: From genes to Applications, D. Drider, S. Rebuffat, Eds. (Springer, 2011), pp. 29–53.
-
- Mathur H., Rea M. C., Cotter P. D., Hill C., Ross R. P., The sactibiotic subclass of bacteriocins: An update. Curr. Protein Pept. Sci. 16, 549–558 (2015). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

