Tissue stiffness at the human maternal-fetal interface
- PMID: 31579915
- PMCID: PMC6809602
- DOI: 10.1093/humrep/dez139
Tissue stiffness at the human maternal-fetal interface
Abstract
Study question: What is the stiffness (elastic modulus) of human nonpregnant secretory phase endometrium, first trimester decidua, and placenta?
Summary answer: The stiffness of decidua basalis, the site of placental invasion, was an order of magnitude higher at 103 Pa compared to 102 Pa for decidua parietalis, nonpregnant endometrium and placenta.
What is known already: Mechanical forces have profound effects on cell behavior, regulating both cell differentiation and migration. Despite their importance, very little is known about their effects on blastocyst implantation and trophoblast migration during placental development because of the lack of mechanical characterization at the human maternal-fetal interface.
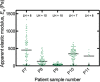
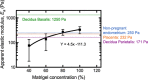
Study design, size, duration: An observational study was conducted to measure the stiffness of ex vivo samples of human nonpregnant secretory endometrium (N = 5) and first trimester decidua basalis (N = 6), decidua parietalis (N = 5), and placenta (N = 5). The stiffness of the artificial extracellular matrix (ECM), Matrigel®, commonly used to study migration of extravillous trophoblast (EVT) in three dimensions and to culture endometrial and placental organoids, was also determined (N = 5).
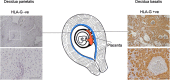
Participants/materials, setting, methods: Atomic force microscopy was used to perform ex vivo direct measurements to determine the stiffness of fresh tissue samples. Decidua was stained by immunohistochemistry (IHC) for HLA-G+ EVT to confirm whether samples were decidua basalis or decidua parietalis. Endometrium was stained with hematoxylin and eosin to confirm the presence of luminal epithelium. Single-cell RNA sequencing data were analyzed to determine expression of ECM transcripts by decidual and placental cells. Fibrillin 1, a protein identified by these data, was stained by IHC in decidua basalis.
Main results and the role of chance: We observed that decidua basalis was significantly stiffer than decidua parietalis, at 1250 and 171 Pa, respectively (P < 0.05). The stiffness of decidua parietalis was similar to nonpregnant endometrium and placental tissue (250 and 232 Pa, respectively). These findings suggest that it is the presence of invading EVT that is driving the increase in stiffness in decidua basalis. The stiffness of Matrigel® was found to be 331 Pa, significantly lower than decidua basalis (P < 0.05).
Large scale data: N/A.
Limitations, reasons for caution: Tissue stiffness was derived by ex vivo measurements on blocks of fresh tissue in the absence of blood flow. The nonpregnant endometrium samples were obtained from women undergoing treatment for infertility. These may not reflect the stiffness of endometrium from normal fertile women.
Wider implications of the findings: These results provide direct measurements of tissue stiffness during the window of implantation and first trimester of human pregnancy. They serve as a basis of future studies exploring the impact of mechanics on embryo implantation and development of the placenta. The findings provide important baseline data to inform matrix stiffness requirements when developing in vitro models of trophoblast stem cell development and migration that more closely resemble the decidua in vivo.
Study funding/competing interest(s): This work was supported by the Centre for Trophoblast Research, the Wellcome Trust (090108/Z/09/Z, 085992/Z/08/Z), the Medical Research Council (MR/P001092/1), the European Research Council (772426), an Engineering and Physical Sciences Research Council Doctoral Training Award (1354760), a UK Medical Research Council and Sackler Foundation Doctoral Training Grant (RG70550) and a Wellcome Trust Doctoral Studentship (215226/Z/19/Z).
Keywords: blastocyst implantation; human; mechanics; tissue stiffness; trophoblast invasion.
© The Author(s) 2019. Published by Oxford University Press on behalf of the European Society of Human Reproduction and Embryology.
Figures


References
-
- Bella J, Hulmes DJS. Fibrillar collagens. Subcell Biochem 2017;82:457–490. - PubMed
-
- Benirschke K, Burton GJ, Baergen RN. Pathology of the Human Placenta, Springer. Berlin Heidelberg, 2012, http://link.springer.com/10.1007/978-3-642-23941-0. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

