Genetic or pharmacologic blockade of enhancer of zeste homolog 2 inhibits the progression of peritoneal fibrosis
- PMID: 31579944
- PMCID: PMC7481741
- DOI: 10.1002/path.5352
Genetic or pharmacologic blockade of enhancer of zeste homolog 2 inhibits the progression of peritoneal fibrosis
Abstract
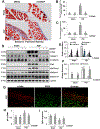
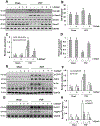
Dysregulation of histone methyltransferase enhancer of zeste homolog 2 (EZH2) has been implicated in the pathogenesis of many cancers. However, the role of EZH2 in peritoneal fibrosis remains unknown. We investigated EZH2 expression in peritoneal dialysis (PD) patients and assessed its role in peritoneal fibrosis in cultured human peritoneal mesothelial cells (HPMCs) and murine models of peritoneal fibrosis induced by chlorhexidine gluconate (CG) or high glucose peritoneal dialysis fluid (PDF) by using 3-deazaneplanocin A (3-DZNeP), and EZH2 conditional knockout mice. An abundance of EZH2 was detected in the peritoneum of patients with PD associated peritonitis and the dialysis effluent of long-term PD patients, which was positively correlated with expression of TGF-β1, vascular endothelial growth factor, and IL-6. EZH2 was found highly expressed in the peritoneum of mice following injury by CG or PDF. In both mouse models, treatment with 3-DZNeP attenuated peritoneal fibrosis and inhibited activation of several profibrotic signaling pathways, including TGF-β1/Smad3, Notch1, epidermal growth factor receptor and Src. EZH2 inhibition also inhibited STAT3 and nuclear factor-κB phosphorylation, and reduced lymphocyte and macrophage infiltration and angiogenesis in the injured peritoneum. 3-DZNeP effectively improved high glucose PDF-associated peritoneal dysfunction by decreasing the dialysate-to-plasma ratio of blood urea nitrogen and increasing the ratio of dialysate glucose at 2 h after PDF injection to initial dialysate glucose. Moreover, delayed administration of 3-DZNeP inhibited peritoneal fibrosis progression, reversed established peritoneal fibrosis and reduced expression of tissue inhibitor of metalloproteinase 2, and matrix metalloproteinase-2 and -9. Finally, EZH2-KO mice exhibited less peritoneal fibrosis than EZH2-WT mice. In HPMCs, treatment with EZH2 siRNA or 3-DZNeP suppressed TGF-β1-induced upregulation of α-SMA and Collagen I and preserved E-cadherin. These results indicate that EZH2 is a key epigenetic regulator that promotes peritoneal fibrosis. Targeting EZH2 may have the potential to prevent and treat peritoneal fibrosis. © 2019 Pathological Society of Great Britain and Ireland. Published by John Wiley & Sons, Ltd.
Keywords: angiogenesis; enhancer of zeste homolog 2; epithelial-to-mesenchymal transition; peritoneal fibrosis; profibrotic signaling pathways.
© 2019 Pathological Society of Great Britain and Ireland. Published by John Wiley & Sons, Ltd.
Figures




References
-
- Zhou Q, Bajo MA, Del Peso G, et al. Preventing peritoneal membrane fibrosis in peritoneal dialysis patients. Kidney Int 2016; 90: 515–524. - PubMed
-
- Yao Q, Pawlaczyk K, Ayala ER, et al. The role of the TGF/Smad signaling pathway in peritoneal fibrosis induced by peritoneal dialysis solutions. Nephron Exp Nephrol 2008; 109: e71–78. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

