A Comprehensive Peptidomic Approach to Characterize the Protein Profile of Selected Durum Wheat Genotypes: Implication for Coeliac Disease and Wheat Allergy
- PMID: 31581419
- PMCID: PMC6835779
- DOI: 10.3390/nu11102321
A Comprehensive Peptidomic Approach to Characterize the Protein Profile of Selected Durum Wheat Genotypes: Implication for Coeliac Disease and Wheat Allergy
Abstract
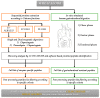
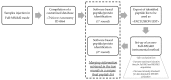
The wheat varietal selection undertaken by breeders in recent decades has been tailored mainly to improve technological and productivity-related traits; however, the latter has resulted in a considerable impoverishment of the genetic diversity of wheat-based products available on the market. This pitfall has encouraged researchers to revalue the natural diversity of cultivated and non-cultivated wheat genotypes in light of their different toxic/immunogenic potential for celiac disease and wheat-allergic patients. In the present investigation, an advanced proteomic approach was designed for the global characterization of the protein profile of selected tetraploid wheat genotypes (Triticum turgidum). The approach combined proteins/peptides sequence information retrieved by specific enzymatic digestions (single and dual proteolytic enzymes) with protein digestibility information disclosed by means of in-vitro simulated human gastroduodenal digestion experiments. In both cases, the peptide pools were characterized by discovery analysis with liquid chromatography high-resolution tandem mass spectrometry, and specific amino acid sequences were identified via commercial software. The peptide list was screened for in silico toxicity/immunogenicity risk assessment, with the aid of various open-source bioinformatics tools for epitopes matching. Given the global information provided by the designed proteomic approach, the in silico risk assessment not only tackled toxicity implication for celiac disease patients, but also scouted for immunogenic sequences relevant for wheat allergic patients, achieving a comprehensive characterization of the protein profile of the selected genotypes. These latter were assessed to encrypt a variable number of toxic/immunogenic epitopes for celiac disease and wheat allergy, and as such they could represent convenient bases for breeding practices and for the development of new detoxification strategies.
Keywords: celiac disease; epitopes; high resolution mass spectrometry; in-vitro gastroduodenal digestion; peptidomic approach; wheat allergy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Alves T.O., D’Almeida C.T.S., Victorio V.C.M., Souz G.H.M.F., Cameron L.C., Ferreira M.S.L. Immunogenic and allergenic profile of wheat flours from different technological qualities revealed by ion mobility mass spectrometry. J. Food Compos. Anal. 2018;73:67–75. doi: 10.1016/j.jfca.2018.07.012. - DOI
-
- Goesaert H., Brijs K., Veraverbeke W.S., Courtin C.M., Gebruers K., Delcour J.A. Wheat flour constituents: How they impact bread quality, and how to impact their functionality. Trends Food Sci. Technol. 2005;16:12–30. doi: 10.1016/j.tifs.2004.02.011. - DOI
-
- D’Ovidio R., Masci S. The low-molecular-weight glutenin subunits of wheat gluten. J. Cereal Sci. 2004;39:321–339. doi: 10.1016/j.jcs.2003.12.002. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

