Anti-influenza hyperimmune intravenous immunoglobulin for adults with influenza A or B infection (FLU-IVIG): a double-blind, randomised, placebo-controlled trial
- PMID: 31582358
- PMCID: PMC6868512
- DOI: 10.1016/S2213-2600(19)30253-X
Anti-influenza hyperimmune intravenous immunoglobulin for adults with influenza A or B infection (FLU-IVIG): a double-blind, randomised, placebo-controlled trial
Abstract
Background: Since the 1918 influenza pandemic, non-randomised studies and small clinical trials have suggested that convalescent plasma or anti-influenza hyperimmune intravenous immunoglobulin (hIVIG) might have clinical benefit for patients with influenza infection, but definitive data do not exist. We aimed to evaluate the safety and efficacy of hIVIG in a randomised controlled trial.
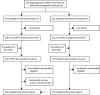
Methods: This randomised, double-blind, placebo-controlled trial was planned for 45 hospitals in Argentina, Australia, Denmark, Greece, Mexico, Spain, Thailand, UK, and the USA over five influenza seasons from 2013-14 to 2017-18. Adults (≥18 years of age) were admitted for hospital treatment with laboratory-confirmed influenza A or B infection and were randomly assigned (1:1) to receive standard care plus either a single 500-mL infusion of high-titre hIVIG (0·25 g/kg bodyweight, 24·75 g maximum; hIVIG group) or saline placebo (placebo group). Eligible patients had a National Early Warning score of 2 points or greater at the time of screening and their symptoms began no more than 7 days before randomisation. Pregnant and breastfeeding women were excluded, as well as any patients for whom the treatment would present a health risk. Separate randomisation schedules were generated for each participating clinical site using permuted block randomisation. Treatment assignments were obtained using a web-based application by the site pharmacist who then masked the solution for infusion. Patients and investigators were masked to study treatment. The primary endpoint was a six-category ordinal outcome of clinical status at day 7, ranging in severity from death to resumption of normal activities after discharge. The choice of day 7 was based on haemagglutination inhibition titres from a pilot study. It was analysed with a proportional odds model, using all six categories to estimate a common odds ratio (OR). An OR greater than 1 indicated that, for a given category, patients in the hIVIG group were more likely to be in a better category than those in the placebo group. Prespecified primary analyses for safety and efficacy were based on patients who received an infusion and for whom eligibility could be confirmed. This trial is registered with ClinicalTrials.gov, NCT02287467.
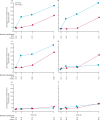
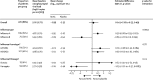
Findings: 313 patients were enrolled in 34 sites between Dec 11, 2014, and May 28, 2018. We also used data from 16 patients enrolled at seven of the 34 sites during the pilot study between Jan 15, 2014, and April 10, 2014. 168 patients were randomly assigned to the hIVIG group and 161 to the placebo group. 21 patients were excluded (12 from the hIVIG group and 9 from the placebo group) because they did not receive an infusion or their eligibility could not be confirmed. Thus, 308 were included in the primary analysis. hIVIG treatment produced a robust rise in haemagglutination inhibition titres against influenza A and smaller rises in influenza B titres. Based on the proportional odds model, the OR on day 7 was 1·25 (95% CI 0·79-1·97; p=0·33). In subgroup analyses for the primary outcome, the OR in patients with influenza A was 0·94 (0·55-1·59) and was 3·19 (1·21-8·42) for those with influenza B (interaction p=0·023). Through 28 days of follow-up, 47 (30%) of 156 patients in the hIVIG group and in 45 (30%) of 152 patients in the placebo group had the composite safety outcome of death, a serious adverse event, or a grade 3 or 4 adverse event (hazard ratio [HR] 1·06, 95% CI 0·70-1·60; p=0·79). Six (4%) patients in the hIVIG group and five (3%) in the placebo group died, but these deaths were not necessarily related to treatment.
Interpretation: When administered alongside standard care (most commonly oseltamivir), hIVIG was not superior to placebo for adults hospitalised with influenza infection. By contrast with our prespecified subgroup hypothesis that hIVIG would result in more favourable responses in patients with influenza A than B, we found the opposite effect. The clinical benefit of hIVIG for patients with influenza B is supported by antibody affinity analyses, but confirmation is warranted.
Funding: NIAID and NIH. Partial support was provided by the Medical Research Council (MRC_UU_12023/23) and the Danish National Research Foundation.
Copyright © 2019 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY-NC-ND 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Figures


Comment in
-
Passive immunity for the treatment of influenza: quality not quantity.Lancet Respir Med. 2019 Nov;7(11):922-923. doi: 10.1016/S2213-2600(19)30265-6. Epub 2019 Sep 30. Lancet Respir Med. 2019. PMID: 31582359 No abstract available.
References
-
- Paules C, Subbarao K. Influenza. Lancet. 2017;390:697–708. - PubMed
-
- Dobson J, Whitley RJ, Pocock S, Monto AS. Oseltamivir treatment for influenza in adults: a meta-analysis of randomized controlled trials. Lancet. 2015;385:1729–1737. - PubMed
-
- Ramirez J, Peyrani P, Wiemken T. A randomized study evaluating the effectiveness of oseltamivir initiated at the time of hospital admission in adults hospitalized with influenza-associated lower respiratory tract infections. Clin Infect Dis. 2018;67:736–742. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

