Anti-influenza immune plasma for the treatment of patients with severe influenza A: a randomised, double-blind, phase 3 trial
- PMID: 31582360
- PMCID: PMC6941345
- DOI: 10.1016/S2213-2600(19)30199-7
Anti-influenza immune plasma for the treatment of patients with severe influenza A: a randomised, double-blind, phase 3 trial
Abstract
Background: Infection with influenza virus causes substantial morbidity and mortality globally, although antiviral treatments are available. Previous studies have suggested that anti-influenza immune plasma could be beneficial as treatment, but they were not designed as randomised, blinded, placebo-controlled trials. Therefore, we aimed to prospectively evaluate the clinical efficacy of high-titre immune plasma compared with standard low-titre plasma to improve outcomes in patients with severe influenza A infection.
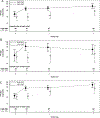
Methods: We did this randomised, double-blind, phase 3 trial at 41 US medical centres to assess the efficacy of high-titre anti-influenza plasma (haemagglutination inhibition antibody titre ≥1:80) compared with low-titre plasma (≤1:10). Children and adults with PCR-confirmed influenza A infection, a National Early Warning score of 3 or greater, and onset of illness within 6 days before randomisation were eligible. Patients were randomly assigned (2:1) using an interactive web response system to receive either two units (or paediatric equivalent) of high-titre plasma (high-titre group) or low-titre plasma (low-titre group), and were followed up for 28 days from randomisation. High-titre and low-titre plasma had the same appearance. Randomisation was stratified by severity (in intensive care unit, not in intensive care but requiring supplemental oxygen, or not in intensive care and not requiring supplemental oxygen) and age (<18 years and ≥18 years). All participants, site staff, and the study team were masked to treatment allocation until after the final database lock. The primary endpoint was clinical status assessed by a six-point ordinal scale on day 7 (death, in intensive care, hospitalised but requiring supplemental oxygen, hospitalised not requiring supplemental oxygen, discharged but unable to resume normal activities, and discharged with full resumption of normal activities) analysed in a proportional odds model (an odds ratio [OR] >1 indicates improvement in clinical status across all categories for the high-titre vs the low-titre group). The primary analysis was done in the intention-to-treat population, excluding two participants who did not receive plasma. This trial is registered with ClinicalTrials.gov, NCT02572817.
Findings: Participants were recruited between Jan 26, 2016, and April 19, 2018. Of 200 participants enrolled (177 adults and 23 children), 140 met the criteria for randomisation and were assigned to the high-titre group (n=92) or to the control low-titre group (n=48). One participant from each group did not receive plasma. At baseline, 60 (43%) of 138 participants were in intensive care and 55 (71%) of 78 participants who were not in intensive care required oxygen. 93% of planned plasma infusions were completed. The study was terminated in July, 2018, when independent efficacy analysis showed low conditional power to detect an effect of high-titre plasma even if full accrual (150 participants) was achieved. The proportional OR for improved clinical status on day 7 was 1·22 (95% CI 0·65-2·29, p=0·54). 47 (34%) of 138 participants experienced 88 serious adverse events: 32 (35%) with 60 events in the high-titre group and 15 (32%) with 28 events in the low-titre group. The most common serious adverse events were acute respiratory distress syndrome (ARDS; four [4%] vs two [4%]), allergic transfusion reactions (two [2%] vs two [4%]), and respiratory distress (three [3%] vs none). 65 (47%) participants experienced 183 adverse events: 42 (46%) with 126 events in the high-titre group and 23 (49%) with 57 events in the low-titre group. The most common adverse events were anaemia (four [3%] vs two [4%]) and ARDS (four [3%] vs three [5%]). Ten patients died during the study (six [7%] in the high-titre group vs four [9%] in the low-titre group, p=0·73). The most common cause of death was worsening of acute respiratory distress syndrome (two [2%] vs two [4%] patients).
Interpretation: High-titre anti-influenza plasma conferred no significant benefit over non-immune plasma. Although our study did not have the precision to rule out a small, clinically relevant effect, the benefit is insufficient to justify the use of immune plasma for treating patients with severe influenza A.
Funding: National Institute of Allergy and Infectious Diseases of the National Institutes of Health (Bethesda, MD, USA).
Copyright © 2019 Elsevier Ltd. All rights reserved.
Figures

Comment in
-
Passive immunity for the treatment of influenza: quality not quantity.Lancet Respir Med. 2019 Nov;7(11):922-923. doi: 10.1016/S2213-2600(19)30265-6. Epub 2019 Sep 30. Lancet Respir Med. 2019. PMID: 31582359 No abstract available.
References
-
- Luke TC, Kilbane EM, Jackson JL, Hoffman SL. Meta-analysis: convalescent blood products for Spanish influenza pneumonia: a future H5N1 treatment? Ann Intern Med 2006; 145(8): 599–609. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

