Hypertension and incident cardiovascular events following ibrutinib initiation
- PMID: 31582362
- PMCID: PMC6887116
- DOI: 10.1182/blood.2019000840
Hypertension and incident cardiovascular events following ibrutinib initiation
Abstract
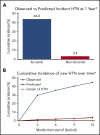
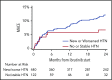
Ibrutinib is associated with dramatic efficacy against B-cell malignancies. Yet, it has been linked with potentially limiting cardiotoxicity, including emerging reports of profound hypertension (HTN). The long-term incidence, severity, and impact of HTN development with ibrutinib are unknown. Therefore, in 562 consecutive patients treated with ibrutinib for B-cell malignancies from 2009 through 2016, we assessed the new/incident or worsened HTN (systolic blood pressure [BP] cutoff, 130 mm Hg). Observed incident HTN rates were compared with Framingham-heart-predicted incident HTN rates. We also evaluated the relationship of HTN to the development of other major adverse cardiovascular events (MACEs), including arrhythmia, myocardial infarction, stroke, heart failure, and cardiovascular death. Further, we assessed the effects of different antihypertensive classes on ibrutinib-related HTN. Overall, 78.3% of ibrutinib users developed new or worsened HTN over a median of 30 months. New HTN developed in 71.6% of ibrutinib users, with a time to 50% cumulative incidence of 4.2 months. Among those without preceding HTN, 17.7% developed high-grade HTN (BP >160/100 mm Hg). In multivariate regression, new or worsened HTN was associated with increased MACEs (hazard ratio [HR], 2.17; 95% confidence interval [CI], 1.08-4.38). No single antihypertensive class was associated with prevention or control of ibrutinib-related HTN. However, antihypertensive initiation was associated with a lower risk of a MACE (HR, 0.40; 95% CI, 0.24-0.66). Collectively, these data suggest that ibrutinib is associated with a substantial increase in the incidence and severity of HTN, and that HTN development carries a higher risk of subsequent cardiotoxic events.
© 2019 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: J.C.B. received research funding and consulted for Acerta Pharma and Pharmacyclics. F.A. received research funding from Innate Pharma and Pharmacyclics; has provided consulting services to Gilead Sciences, Pharmacyclics, Janssen, Abbvie, Sunesis, AstraZeneca, Genentech, and Novartis Oncology; and has served on the speakers’ bureau of Abbvie and AstraZeneca. K.A.R. received research funding from Genentech and served on an advisory board for Acerta Pharma. J.A.W. received research funding from Abbvie, Pharmacyclics, Janssen, Acerta, Loxo, Karyopharm, and Morphosys and has consulted for Janssen and Pharmacyclics. The remaining authors declare no competing financial interests.
Figures


Comment in
-
Cardiovascular adverse events of ibrutinib.Blood. 2019 Nov 28;134(22):1881-1882. doi: 10.1182/blood.2019002805. Blood. 2019. PMID: 31778540 Free PMC article.
References
-
- Treon SP, Tripsas CK, Meid K, et al. . Ibrutinib in previously treated Waldenström’s macroglobulinemia. N Engl J Med. 2015;372(15):1430-1440. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

