ILC2s mediate systemic innate protection by priming mucus production at distal mucosal sites
- PMID: 31582416
- PMCID: PMC6888984
- DOI: 10.1084/jem.20180610
ILC2s mediate systemic innate protection by priming mucus production at distal mucosal sites
Abstract
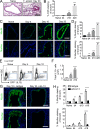
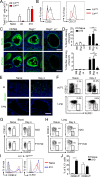
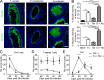
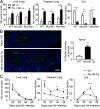
Host immunity to parasitic nematodes requires the generation of a robust type 2 cytokine response, characterized by the production of interleukin 13 (IL-13), which drives expulsion. Here, we show that infection with helminths in the intestine also induces an ILC2-driven, IL-13-dependent goblet cell hyperplasia and increased production of mucins (Muc5b and Muc5ac) at distal sites, including the lungs and other mucosal barrier sites. Critically, we show that type 2 priming of lung tissue through increased mucin production inhibits the progression of a subsequent lung migratory helminth infection and limits its transit through the airways. These data show that infection by gastrointestinal-dwelling helminths induces a systemic innate mucin response that primes peripheral barrier sites for protection against subsequent secondary helminth infections. These data suggest that innate-driven priming of mucus barriers may have evolved to protect from subsequent infections with multiple helminth species, which occur naturally in endemic areas.
© 2019 Campbell et al.
Figures
References
-
- Barlow J.L., Bellosi A., Hardman C.S., Drynan L.F., Wong S.H., Cruickshank J.P., and McKenzie A.N.. 2012. Innate IL-13-producing nuocytes arise during allergic lung inflammation and contribute to airways hyperreactivity. J. Allergy Clin. Immunol. 129:191–8.e1: 4. 10.1016/j.jaci.2011.09.041 - DOI - PubMed
-
- Behnke J.M., Wahid F.N., Grencis R.K., Else K.J., Ben-Smith A.W., and Goyal P.K.. 1993. Immunological relationships during primary infection with Heligmosomoides polygyrus (Nematospiroides dubius): downregulation of specific cytokine secretion (IL-9 and IL-10) correlates with poor mastocytosis and chronic survival of adult worms. Parasite Immunol. 15:415–421. 10.1111/j.1365-3024.1993.tb00626.x - DOI - PubMed
-
- Camberis M., Le Gros G., and Urban J.. 2003. Animal Model of Nippostrongylus brasiliensis and Heligmosomoides polygyrus. Curr. Protoc. Immunol. Chapter 19:Unit 19.12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

