Lenalidomide-based induction and maintenance in elderly newly diagnosed multiple myeloma patients: updated results of the EMN01 randomized trial
- PMID: 31582542
- PMCID: PMC7327625
- DOI: 10.3324/haematol.2019.226407
Lenalidomide-based induction and maintenance in elderly newly diagnosed multiple myeloma patients: updated results of the EMN01 randomized trial
Abstract
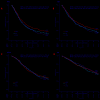
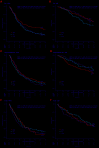
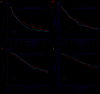
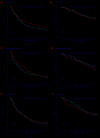
n the EMN01 trial, the addition of an alkylator (melphalan or cyclophosphamide) to lenalidomide-steroid induction therapy was prospectively evaluated in transplant-ineligible patients with multiple myeloma. After induction, patients were randomly assigned to maintenance treatment with lenalidomide alone or with prednisone continuously. The analysis presented here (median follow-up of 71 months) is focused on maintenance treatment and on subgroup analyses defined according to the International Myeloma Working Group Frailty Score. Of the 654 evaluable patients, 217 were in the lenalidomide-dexamethasone arm, 217 in the melphalan-prednisone-lenalidomide arm and 220 in the cyclophosphamide-prednisone-lenalidomide arm. With regards to the Frailty Score, 284 (43%) patients were fit, 205 (31%) were intermediate-fit and 165 (25%) were frail. After induction, 402 patients were eligible for maintenance therapy (lenalidomide arm, n=204; lenalidomide-prednisone arm, n=198). After a median duration of maintenance of 22.0 months, progression-free survival from the start of maintenance was 22.2 months with lenalidomide-prednisone vs 18.6 months with lenalidomide (hazard ratio 0.85, P=0.14), with no differences across frailty subgroups. The most frequent grade ≥3 toxicity was neutropenia (10% of lenalidomide-prednisone and 21% of lenalidomide patients; P=0.001). Grade ≥3 non-hematologic adverse events were rare (<15%). In fit patients, melphalan-prednisone-lenalidomide significantly prolonged progression-free survival compared to cyclophosphamide-prednisone-lenalidomide (hazard ratio 0.72, P=0.05) and lenalidomide-dexamethasone (hazard ratio 0.72, P=0.04). Likewise, a trend towards a better overall survival was noted for patients treated with melphalan-prednisone-lenalidomide or cyclophosphamide-prednisone-lenalidomide, as compared to lenalidomide-dexamethasone. No differences were observed in intermediate-fit and frail patients. This analysis showed positive outcomes of maintenance with lenalidomide-based regimens, with a good safety profile. For the first time, we showed that fit patients benefit from a full-dose triplet regimen, while intermediate-fit and frail patients benefit from gentler regimens. ClinicalTrials.gov registration number: NCT01093196.
Copyright© 2020 Ferrata Storti Foundation.
Figures
References
-
- Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2011;364(11):1046-1060. - PubMed
-
- Palumbo A, Bringhen S, Caravita T, et al. Oral melphalan and prednisone chemotherapy plus thalidomide compared with melphalan and prednisone alone in elderly patients with multiple myeloma: randomised controlled trial. Lancet. 2006;367(9513):825-831. - PubMed
-
- San Miguel JF, Schlag R, Khuageva NK, et al. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med. 2008;359(9):906-917. - PubMed
-
- Benboubker L, Dimopoulos MA, Dispenzieri A, et al. Lenalidomide and dexamethasone in transplant-ineligible patients with myeloma. N Engl J Med. 2014;371(10):906-917. - PubMed
-
- Palumbo A, Hajek R, Delforge M, et al. Continuous lenalidomide treatment for newly diagnosed multiple myeloma. N Engl J Med. 2012;366(19):1759-1769. - PubMed

