Inhibition of Synovial Macrophage Pyroptosis Alleviates Synovitis and Fibrosis in Knee Osteoarthritis
- PMID: 31582897
- PMCID: PMC6754937
- DOI: 10.1155/2019/2165918
Inhibition of Synovial Macrophage Pyroptosis Alleviates Synovitis and Fibrosis in Knee Osteoarthritis
Abstract
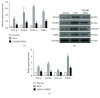
Increasing evidence has shown that macrophage pyroptosis in different tissues participates in chronic aseptic inflammation and is related to tissue fibrosis. Our last studies also revealed the vital role of synovial fibroblast pyroptosis in the onset and development of knee osteoarthritis (KOA). In this study, we aimed to investigate whether synovial macrophage pyroptosis did occur and whether this form of cell death should be related to synovitis and fibrosis of KOA. In the synovial tissue of KOA model rats, we observed a decrease of caspase1, NLRP3, ASC, and GSDMD caused by macrophage depletion in both the mRNA and protein expressions. Besides, rats treated with the specific caspase1 inhibitor Ac-YVAD-CMK showed less inflammatory reaction and fibrosis, not only in the expression of proinflammatory factors IL-1β, IL-18, and HMGB1 and fibrosis markers TGF-β, PLOD2, COL1A1, and TIMP1 but also in the observation of HE staining, Sirius Red staining, and the transverse diameters of the right knees. Subsequently, we established an LPS+ATP-induced model in macrophages mimicking the inflammatory environment of KOA and inducing macrophage pyroptosis. Macrophages transfected with caspase1 siRNA showed reduced cell death; meanwhile, the relative expression of pyroptosis-related proteins were also downregulated. In addition, the level of fibrotic markers in synovial fibroblasts were significantly decreased after coculture with siRNA GSDMD-transfected macrophages. To conclude, synovial macrophage pyroptosis may occur in the pathological processes of KOA and inhibition of synovial macrophage pyroptosis alleviates synovitis and fibrosis in KOA model rats.
Copyright © 2019 Li Zhang et al.
Conflict of interest statement
All authors declare that there are no actual or potential conflicts of interest, including any financial, personal, or other relationships with other people or organizations that could inappropriately influence, or be perceived to influence, our work.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

