The Role of Autologous Dermal Micrografts in Regenerative Surgery: A Clinical Experimental Study
- PMID: 31582991
- PMCID: PMC6754962
- DOI: 10.1155/2019/9843407
The Role of Autologous Dermal Micrografts in Regenerative Surgery: A Clinical Experimental Study
Abstract
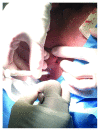
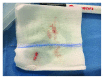
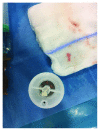
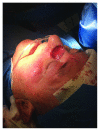
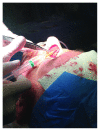
The aim of the study was the objective assessment of the effectiveness of a microfragmented dermal extract obtained with Rigenera™ technology in promoting the wound healing process in an in vivo homogeneous experimental human acute surgical wound model. The study included 20 patients with 24 acute postsurgical soft tissue loss and a planned sequential two-stage repair with a dermal substitute and an autologous split-thickness skin graft. Each acute postsurgical soft tissue loss was randomized to be treated either with an Integra® dermal substitute enriched with the autologous dermal micrografts obtained with Rigenera™ technology (group A-Rigenera™ protocol) or with an Integra® dermal substitute only (group B-control). The reepithelialization rate in the wounds was assessed in both groups at 4 weeks through digital photography with the software "ImageJ." The dermal cell suspension enrichment with the Rigenera™ technology was considered effective if the reepithelialized area was higher than 25% of the total wound surface as this threshold was considered far beyond the expected spontaneous reepithelialization rate. In the Rigenera™ protocol group, the statistical analysis failed to demonstrate any significant difference vs. the controls. The old age of the patients likely influenced the outcome as the stem cell regenerative potential is reduced in the elderly. A further explanation for the unsatisfying results of our trial might be the inadequate amount of dermal stem cells used to enrich the dermal substitutes. In our study, we used a 1 : 200 donor/recipient site ratio to minimize donor site morbidity. The gross dimensional disparity between the donor and recipient sites and the low concentration of dermal mesenchymal stromal stem cells might explain the poor epithelial proliferative boost observed in our study. A potential option in the future might be preconditioning of the dermal stem cell harvest with senolytic active principles that would fully enhance their regenerative potential. This trial is registered with trial protocol number NCT03912675.
Copyright © 2019 Marco Mario Tresoldi et al.
Conflict of interest statement
The author Antonio Graziano is a member of the Scientific Division of Human Brain Wave, the company owner of Rigenera™ Technology. The other authors have no conflict of interests to declare.
Figures



References
-
- Baglioni E., Trovato L., Marcarelli M., Frenello A., Bocchiotti M. A. Treatment of oncological post-surgical wound dehiscence with autologous skin micrografts. Anticancer Research. 2016;36(3):975–979. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical

