Evolution of Neuroglia
- PMID: 31583583
- PMCID: PMC7188604
- DOI: 10.1007/978-981-13-9913-8_2
Evolution of Neuroglia
Abstract
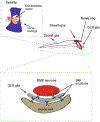
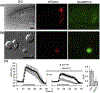
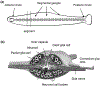
As the nervous system evolved from the diffused to centralised form, the neurones were joined by the appearance of the supportive cells, the neuroglia. Arguably, these non-neuronal cells evolve into a more diversified cell family than the neurones are. The first ancestral neuroglia appeared in flatworms being mesenchymal in origin. In the nematode C. elegans proto-astrocytes/supportive glia of ectodermal origin emerged, albeit the ensheathment of axons by glial cells occurred later in prawns. The multilayered myelin occurred by convergent evolution of oligodendrocytes and Schwann cells in vertebrates above the jawless fishes. Nutritive partitioning of the brain from the rest of the body appeared in insects when the hemolymph-brain barrier, a predecessor of the blood-brain barrier was formed. The defensive cellular mechanism required specialisation of bona fide immune cells, microglia, a process that occurred in the nervous system of leeches, bivalves, snails, insects and above. In ascending phylogeny, new type of glial cells, such as scaffolding radial glia, appeared and as the bran sizes enlarged, the glia to neurone ratio increased. Humans possess some unique glial cells not seen in other animals.
Keywords: Astrocytes; Blood/haemolymph-brain barrier; Brain size; Complexity of glia; Glia to neuron ratio; Microglia; Myelination; Oligodendrocytes; Radial glia.
Figures


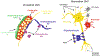



References
-
- Ari C, Kalman M (2008) Evolutionary changes of astroglia in Elasmobranchii comparing to amniotes: a study based on three immunohistochemical markers (GFAP, S-100, and glutamine synthetase). Brain Behav Evol 71:305–324 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

