Physiology of Astroglia
- PMID: 31583584
- PMCID: PMC7265998
- DOI: 10.1007/978-981-13-9913-8_3
Physiology of Astroglia
Abstract
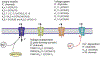
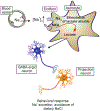
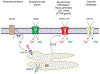
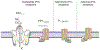
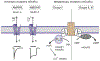
Astrocytes are principal cells responsible for maintaining the brain homeostasis. Additionally, these glial cells are also involved in homocellular (astrocyte-astrocyte) and heterocellular (astrocyte-other cell types) signalling and metabolism. These astroglial functions require an expression of the assortment of molecules, be that transporters or pumps, to maintain ion concentration gradients across the plasmalemma and the membrane of the endoplasmic reticulum. Astrocytes sense and balance their neurochemical environment via variety of transmitter receptors and transporters. As they are electrically non-excitable, astrocytes display intracellular calcium and sodium fluctuations, which are not only used for operative signalling but can also affect metabolism. In this chapter we discuss the molecules that achieve ionic gradients and underlie astrocyte signalling.
Keywords: Astrocytes; Brain homoeostasis; Ca2+ signalling; Ion channels; Na+ signalling; Neurotransmitter receptors; SLC transporters.
Figures




References
-
- Agulhon C, Fiacco TA, McCarthy KD (2010) Hippocampal short- and long-term plasticity are not modulated by astrocyte Ca2+ signaling. Science 327:1250–1254 - PubMed
-
- Allbritton NL, Meyer T, Stryer L (1992) Range of messenger action of calcium ion and inositol 1,4,5-trisphosphate. Science 258:1812–1815 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

