The influence of stomatal morphology and distribution on photosynthetic gas exchange
- PMID: 31583771
- PMCID: PMC7065165
- DOI: 10.1111/tpj.14560
The influence of stomatal morphology and distribution on photosynthetic gas exchange
Abstract
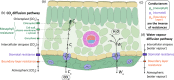
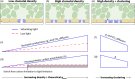
The intricate and interconnecting reactions of C3 photosynthesis are often limited by one of two fundamental processes: the conversion of solar energy into chemical energy, or the diffusion of CO2 from the atmosphere through the stomata, and ultimately into the chloroplast. In this review, we explore how the contributions of stomatal morphology and distribution can affect photosynthesis, through changes in gaseous exchange. The factors driving this relationship are considered, and recent results from studies investigating the effects of stomatal shape, size, density and patterning on photosynthesis are discussed. We suggest that the interplay between stomatal gaseous exchange and photosynthesis is complex, and that a disconnect often exists between the rates of CO2 diffusion and photosynthetic carbon fixation. The mechanisms that allow for substantial reductions in maximum stomatal conductance without affecting photosynthesis are highly dependent on environmental factors, such as light intensity, and could be exploited to improve crop performance.
Keywords: Arabidopsis thaliana; carbon dioxide; development; diffusion; distribution; gaseous exchange; morphology; photosynthesis; stomata.
© 2019 The Authors. The Plant Journal published by Society for Experimental Biology and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Assmann, S.M. and Zeiger, E. (1987) Guard cell bioenergetics In Stomatal Function (Zeiger E., Farquhar G. and Cowan I., eds). Stanford, CA: Stanford Univ. Press, pp. 163–194.
-
- Burt‐Utley, K. and Utley, J.F. (1999) Contributions toward a revision of Begonia section Weilbachia (Begoniaceae). Novon, 9, 483–489.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

