Effect of Seasonal Malaria Chemoprevention on Immune Markers of Exhaustion and Regulation
- PMID: 31584094
- PMCID: PMC6910875
- DOI: 10.1093/infdis/jiz415
Effect of Seasonal Malaria Chemoprevention on Immune Markers of Exhaustion and Regulation
Abstract
Background: Seasonal malaria chemoprevention (SMC) is a novel strategy to reduce malaria infections in children. Infection with Plasmodium falciparum results in immune dysfunction characterized by elevated expression of markers associated with exhaustion, such as PD1 and LAG3, and regulatory CD4+FOXP3+ T cells.
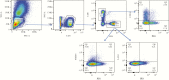
Methods: In the current study, the impact of seasonal malaria chemoprevention on malaria-induced immune dysfunction, as measured by markers associated with exhaustion and regulatory T cells, was explored by flow cytometry.
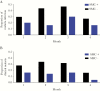
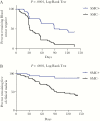
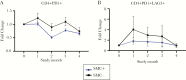
Results: Children that received seasonal malaria chemoprevention had fewer malaria episodes and showed significantly lower fold changes in CD4+PD1+ and CD4+PD1+LAG3+ compared to those that did not receive SMC. Seasonal malaria chemoprevention had no observable effect on fold changes in CD8 T cells expressing PD1 or CD160. However, children receiving SMC showed greater increases in CD4+FOXP3+ T regulatory cells compared to children not receiving SMC.
Conclusions: These results provide important insights into the dynamics of malaria-induced changes in the CD4 T-cell compartment of the immune system and suggest that the reduction of infections due to seasonal malaria chemoprevention may also prevent immune dysfunction.
Clinical trials registration: NCT02504918.
Keywords: T-cell exhaustion; T-cell regulation; immune dysfunction; seasonal malaria chemoprevention.
Published by Oxford University Press for the Infectious Diseases Society of America 2019.
Figures
References
-
- World Health Organization. World Malaria report 2018. Geneva: WHO, 2018.
-
- World Health Organization. Seasonal malaria chemoprevention with sulfadoxine-pyrimethamine plus amodiaquine in children: a field guide. Geneva: WHO, 2013.
-
- Dicko A, Diallo AI, Tembine I, et al. . Intermittent preventive treatment of malaria provides substantial protection against malaria in children already protected by an insecticide-treated bednet in Mali: a randomised, double-blind, placebo-controlled trial. PLoS Med 2011; 8:e1000407. - PMC - PubMed
-
- Langhorne J, Ndungu FM, Sponaas AM, Marsh K. Immunity to malaria: more questions than answers. Nat Immunol 2008; 9:725–32. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

