Elucidating Critical Proteinopathic Mechanisms and Potential Drug Targets in Neurodegeneration
- PMID: 31584139
- PMCID: PMC11449027
- DOI: 10.1007/s10571-019-00741-0
Elucidating Critical Proteinopathic Mechanisms and Potential Drug Targets in Neurodegeneration
Abstract
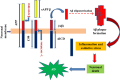
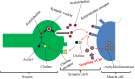
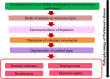
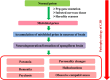
Neurodegeneration entails progressive loss of neuronal structure as well as function leading to cognitive failure, apathy, anxiety, irregular body movements, mood swing and ageing. Proteomic dysregulation is considered the key factor for neurodegeneration. Mechanisms involving deregulated processing of proteins such as amyloid beta (Aβ) oligomerization; tau hyperphosphorylation, prion misfolding; α-synuclein accumulation/lewy body formation, chaperone deregulation, acetylcholine depletion, adenosine 2A (A2A) receptor hyperactivation, secretase deregulation, leucine-rich repeat kinase 2 (LRRK2) mutation and mitochondrial proteinopathies have deeper implications in neurodegenerative disorders. Better understanding of such pathological mechanisms is pivotal for exploring crucial drug targets. Herein, we provide a comprehensive outlook about the diverse proteomic irregularities in Alzheimer's, Parkinson's and Creutzfeldt Jakob disease (CJD). We explicate the role of key neuroproteomic drug targets notably Aβ, tau, alpha synuclein, prions, secretases, acetylcholinesterase (AchE), LRRK2, molecular chaperones, A2A receptors, muscarinic acetylcholine receptors (mAchR), N-methyl-D-aspartate receptor (NMDAR), glial cell line-derived neurotrophic factor (GDNF) family ligands (GFLs) and mitochondrial/oxidative stress-related proteins for combating neurodegeneration and associated cognitive and motor impairment. Cross talk between amyloidopathy, synucleinopathy, tauopathy and several other proteinopathies pinpoints the need to develop safe therapeutics with ability to strike multiple targets in the aetiology of the neurodegenerative disorders. Therapeutics like microtubule stabilisers, chaperones, kinase inhibitors, anti-aggregation agents and antibodies could serve promising regimens for treating neurodegeneration. However, drugs should be target specific, safe and able to penetrate blood-brain barrier.
Keywords: Chaperones; Drug targets; Free radicals; Mitophagy; Neurodegeneration.
Conflict of interest statement
Authors declare that there are no conflict of interest.
Figures








References
-
- Abreu PA, Castro HC, Paes-de-Carvalho R et al (2013) Molecular modeling of a phenylamidine class of NMDA receptor antagonists and the rational design of new triazolyl-amidine derivatives. Chem Biol Drug Des 81:185–197 - PubMed
-
- Adolfsson O, Pihlgren M, Toni N, Varisco Y, Buccarello AL, Antoniello K, Lohmann S, Piorkowska K, Gafner V, Atwal JK, Maloney J (2012) An effector- reduced anti- β-amyloid (Aβ) antibody with unique Aβ binding properties promotes neuroprotection and glial engulfment of Aβ. J Neurosci 32:9677–9789 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

