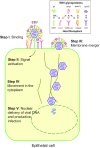
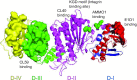
Epithelial cell infection by Epstein-Barr virus
- PMID: 31584659
- PMCID: PMC7317989
- DOI: 10.1093/femsre/fuz023
Epithelial cell infection by Epstein-Barr virus
Abstract
Epstein-Barr Virus (EBV) is etiologically associated with multiple human malignancies including Burkitt lymphoma and Hodgkin disease as well as nasopharyngeal and gastric carcinoma. Entry of EBV into target cells is essential for virus to cause disease and is mediated by multiple viral envelope glycoproteins and cell surface associated receptors. The target cells of EBV include B cells and epithelial cells. The nature and mechanism of EBV entry into these cell types are different, requiring different glycoprotein complexes to bind to specific receptors on the target cells. Compared to the B cell entry mechanism, the overall mechanism of EBV entry into epithelial cells is less well known. Numerous receptors have been implicated in this process and may also be involved in additional processes of EBV entry, transport, and replication. This review summarizes EBV glycoproteins, host receptors, signal molecules and transport machinery that are being used in the epithelial cell entry process and also provides a broad view for related herpesvirus entry mechanisms.
Keywords: Epstein–Barr virus; epithelial cells; gamma-herpesviruses.
© FEMS 2019.
Figures