Association of Histologic Disease Activity With Progression of Nonalcoholic Fatty Liver Disease
- PMID: 31584681
- PMCID: PMC6784786
- DOI: 10.1001/jamanetworkopen.2019.12565
Association of Histologic Disease Activity With Progression of Nonalcoholic Fatty Liver Disease
Abstract
Importance: The histologic evolution of the full spectrum of nonalcoholic fatty liver disease (NAFLD) and factors associated with progression or regression remain to be definitively established.
Objective: To evaluate the histologic evolution of NAFLD and the factors associated with changes in disease severity over time.
Design, setting, and participants: A prospective cohort substudy from the Nonalcoholic Steatohepatitis Clinical Research Network (NASH CRN) NAFLD Database study, a noninterventional registry, was performed at 8 university medical research centers. Masked assessment of liver histologic specimens was performed, using a prespecified protocol to score individual biopsies. Participants included 446 adults with NAFLD enrolled in the NASH CRN Database studies between October 27, 2004, and September 13, 2013, who underwent 2 liver biopsies 1 or more year apart. Data analysis was performed from October 2016 to October 2018.
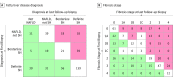
Main outcomes and measures: Progression and regression of fibrosis stage, using clinical, laboratory, and histologic findings, including the NAFLD activity score (NAS) (sum of scores for steatosis, lobular inflammation, and ballooning; range, 0-8, with 8 indicating more severe disease).
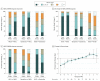
Results: A total of 446 adults (mean [SD] age, 47 [11] years; 294 [65.9%] women) with NAFLD (NAFL, 86 [19.3%]), borderline NASH (84 [18.8%]), and definite NASH (276 [61.9%]) were studied. Over a mean (SD) interval of 4.9 (2.8) years between biopsies, NAFL resolved in 11 patients (12.8%) and progressed to steatohepatitis in 36 patients (41.9%). Steatohepatitis resolved in 24 (28.6%) of the patients with borderline NASH and 61 (22.1%) of those with definite NASH. Fibrosis progression or regression by at least 1 stage occurred in 132 (30%) and 151 [34%] participants, respectively. Metabolic syndrome (20 [95%] vs 108 [72%]; P = .03), baseline NAS (mean [SD], 5.0 [1.4] vs 4.3 [1.6]; P = .005), and smaller reduction in NAS (-0.2 [2] vs -0.9 [2]; P < .001) were associated with progression to advanced (stage 3-4) fibrosis vs those without progression to stage 3 to 4 fibrosis. Fibrosis regression was associated with lower baseline insulin level (20 vs 33 μU/mL; P = .02) and decrease in all NAS components (steatosis grade -0.8 [0.1] vs -0.3 [0.9]; P < .001; lobular inflammation -0.5 [0.8] vs -0.2 [0.9]; P < .001; ballooning -0.7 [1.1] vs -0.1 [0.9]; P < .001). Only baseline aspartate aminotransferase (AST) levels were associated with fibrosis regression vs no change and progression vs no change on multivariable regression: baseline AST (regression: conditional odds ratio [cOR], 0.6 per 10 U/L AST; 95% CI, 0.4-0.7; P < .001; progression: cOR, 1.3; 95% CI, 1.1-1.5; P = .002). Changes in the AST level, alanine aminotransferase (ALT) level, and NAS were also associated with fibrosis regression and progression (ΔAST level: regression, cOR, 0.9; 95% CI, 0.6-1.2; P = .47; progression, cOR, 1.3; 95% CI, 1.0-1.6; P = .02; ΔALT level: regression, cOR, 0.7 per 10 U/L AST; 95% CI, 0.5-0.9; P = .002; progression, cOR, 1.0 per 10 U/L AST; 95% CI, 0.9-1.2; P = .93; ΔNAS: regression, cOR, 0.7; 95% CI, 0.6-0.9; P = .001; progression, cOR, 1.3; 95% CI, 1.1-1.5; P = .01).
Conclusions and relevance: Improvement or worsening of disease activity may be associated with fibrosis regression or progression, respectively, in NAFLD.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
- U01 DK061731/DK/NIDDK NIH HHS/United States
- UL1 TR000006/TR/NCATS NIH HHS/United States
- UL1 TR000058/TR/NCATS NIH HHS/United States
- UL1 TR000436/TR/NCATS NIH HHS/United States
- U01 DK061728/DK/NIDDK NIH HHS/United States
- UL1 TR000439/TR/NCATS NIH HHS/United States
- UL1 TR002319/TR/NCATS NIH HHS/United States
- U01 DK061734/DK/NIDDK NIH HHS/United States
- U01 DK061737/DK/NIDDK NIH HHS/United States
- U01 DK061713/DK/NIDDK NIH HHS/United States
- U01 DK061732/DK/NIDDK NIH HHS/United States
- UL1 TR002548/TR/NCATS NIH HHS/United States
- U01 DK061718/DK/NIDDK NIH HHS/United States
- UL1 TR001442/TR/NCATS NIH HHS/United States
- U01 DK061730/DK/NIDDK NIH HHS/United States
- U24 DK061730/DK/NIDDK NIH HHS/United States
- U01 DK061738/DK/NIDDK NIH HHS/United States
- UL1 TR000448/TR/NCATS NIH HHS/United States
- UL1 TR000423/TR/NCATS NIH HHS/United States
- UL1 TR000100/TR/NCATS NIH HHS/United States
- UL1 TR002345/TR/NCATS NIH HHS/United States
- UL1 TR000004/TR/NCATS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

