Sample Size Estimates for Cluster-Randomized Trials in Hospital Infection Control and Antimicrobial Stewardship
- PMID: 31584684
- PMCID: PMC6784749
- DOI: 10.1001/jamanetworkopen.2019.12644
Sample Size Estimates for Cluster-Randomized Trials in Hospital Infection Control and Antimicrobial Stewardship
Abstract
Importance: An important step in designing, executing, and evaluating cluster-randomized trials (CRTs) is understanding the correlation and thus nonindependence that exists among individuals in a cluster. In hospital epidemiology, there is a shortage of CRTs that have published their intraclass correlation coefficient or coefficient of variation (CV), making prospective sample size calculations difficult for investigators.
Objectives: To estimate the number of hospitals needed to power parallel CRTs of interventions to reduce health care-associated infection outcomes and to demonstrate how different parameters such as CV and expected effect size are associated with the sample size estimates in practice.
Design, setting, and participants: This longitudinal cohort study estimated parameters for sample size calculations using national rates developed by the Centers for Disease Control and Prevention for methicillin-resistant Staphylococcus aureus (MRSA) bacteremia, central-line-associated bloodstream infections (CLABSI), catheter-associated urinary tract infections (CAUTI), and Clostridium difficile infections (CDI) from 2016. For MRSA and vancomycin-resistant enterococci (VRE) acquisition, outcomes were estimated using data from 2012 from the Benefits of Universal Glove and Gown study. Data were collected from June 2017 through September 2018 and analyzed from September 2018 through January 2019.
Main outcomes and measures: Calculated number of clusters needed for adequate power to detect an intervention effect using a 2-group parallel CRT.
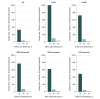
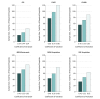
Results: To study an intervention with a 30% decrease in daily rates, 73 total clusters were needed (37 in the intervention group and 36 in the control group) for MRSA bacteremia, 82 for CAUTI, 60 for CLABSI, and 31 for CDI. If a 10% decrease in rates was expected, 768 clusters were needed for MRSA bacteremia, 875 for CAUTI, 631 for CLABSI, and 329 for CDI. For MRSA or VRE acquisition, 50 or 40 total clusters, respectively, were required to observe a 30% decrease, whereas 540 or 426 clusters, respectively, were required to detect a 10% decrease.
Conclusions and relevance: This study suggests that large sample sizes are needed to appropriately power parallel CRTs targeting infection prevention outcomes. Sample sizes are most associated with expected effect size and CV of hospital rates.
Conflict of interest statement
Figures

References
-
- Centers for Disease Control and Prevention Healthcare-associated infections. https://www.cdc.gov/hai/surveillance/index.html. Reviewed October 5, 2018. Accessed January 9, 2018.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

