Incidence and predictors of retreatment in chronic hepatitis B patients after discontinuation of entecavir or tenofovir treatment
- PMID: 31584951
- PMCID: PMC6777800
- DOI: 10.1371/journal.pone.0222221
Incidence and predictors of retreatment in chronic hepatitis B patients after discontinuation of entecavir or tenofovir treatment
Abstract
Background: This study investigated the incidence and predictors of retreatment after discontinuation of either entecavir (ETV) or tenofovir disoproxil fumarate (TDF) treatment in Taiwan.
Methods: A total of 535 non-cirrhotic chronic hepatitis B (CHB) patients undergoing either ETV (n = 358) or TDF (n = 177) treatment were enrolled. Patients were followed for at least 12 months after stopping ETV or TDF treatment. Most patients (86.3%) fulfilled the retreatment criteria of Taiwan's National Health Plan.
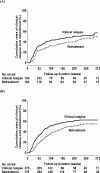
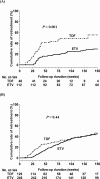
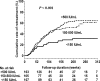
Results: The 5-year cumulative rates of clinical relapse and retreatment were 52.1% and 47%, respectively, in 160 hepatitis B e antigen (HBeAg)-positive patients, and were 62% and 54.8%, respectively, in 375 HBeAg-negative patients. The median duration from the end of treatment until clinical relapse and retreatment was 40 and 57 weeks, respectively, for all patients. Multivariate Cox regression analysis revealed that discontinuing TDF treatment, old age, male gender, and higher baseline HBsAg levels were independent factors of retreatment in HBeAg-positive patients; old age, HBV genotype B, and higher baseline and end-of-treatment HBsAg levels were independent factors in HBeAg-negative patients. A total of 18.8% of retreated patients satisfied the retreatment criteria of hepatic decompensation according to Taiwan's National Health Plan. Of the 64 patients who had clinical relapse without retreatment, 17 achieved sustained virological remission and 26 did not experience clinical relapse until their last visit after clinical relapse. Four patients developed HBsAg loss.
Conclusions: The 5-year retreatment rate was about 50% in HBeAg-positive and HBeAg-negative patients. Discontinuing TDF treatment was an independent factor of retreatment in HBeAg-positive patients.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Weinbaum CM, Mast EE, Ward JW. Recommendations for Identification and Public Health Management of Persons with Chronic Hepatitis B Virus Infection. Hepatology 2009;49:535–544. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

