The efficacy of dihydroartemisinin-piperaquine and artemether-lumefantrine with and without primaquine on Plasmodium vivax recurrence: A systematic review and individual patient data meta-analysis
- PMID: 31584960
- PMCID: PMC6777759
- DOI: 10.1371/journal.pmed.1002928
The efficacy of dihydroartemisinin-piperaquine and artemether-lumefantrine with and without primaquine on Plasmodium vivax recurrence: A systematic review and individual patient data meta-analysis
Abstract
Background: Artemisinin-based combination therapy (ACT) is recommended for uncomplicated Plasmodium vivax malaria in areas of emerging chloroquine resistance. We undertook a systematic review and individual patient data meta-analysis to compare the efficacies of dihydroartemisinin-piperaquine (DP) and artemether-lumefantrine (AL) with or without primaquine (PQ) on the risk of recurrent P. vivax.
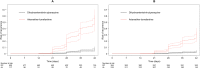
Methods and findings: Clinical efficacy studies of uncomplicated P. vivax treated with DP or AL and published between January 1, 2000, and January 31, 2018, were identified by conducting a systematic review registered with the International Prospective Register of Systematic Reviews (PROSPERO): CRD42016053310. Investigators of eligible studies were invited to contribute individual patient data that were pooled using standardised methodology. The effect of mg/kg dose of piperaquine/lumefantrine, ACT administered, and PQ on the rate of P. vivax recurrence between days 7 and 42 after starting treatment were investigated by Cox regression analyses according to an a priori analysis plan. Secondary outcomes were the risk of recurrence assessed on days 28 and 63. Nineteen studies enrolling 2,017 patients were included in the analysis. The risk of recurrent P. vivax at day 42 was significantly higher in the 384 patients treated with AL alone (44.0%, 95% confidence interval [CI] 38.7-49.8) compared with the 812 patients treated with DP alone (9.3%, 95% CI 7.1-12.2): adjusted hazard ratio (AHR) 12.63 (95% CI 6.40-24.92), p < 0.001. The rates of recurrence assessed at days 42 and 63 were associated inversely with the dose of piperaquine: AHRs (95% CI) for every 5-mg/kg increase 0.63 (0.48-0.84), p = 0.0013 and 0.83 (0.73-0.94), p = 0.0033, respectively. The dose of lumefantrine was not significantly associated with the rate of recurrence (1.07 for every 5-mg/kg increase, 95% CI 0.99-1.16, p = 0.0869). In a post hoc analysis, in patients with symptomatic recurrence after AL, the mean haemoglobin increased 0.13 g/dL (95% CI 0.01-0.26) for every 5 days that recurrence was delayed, p = 0.0407. Coadministration of PQ reduced substantially the rate of recurrence assessed at day 42 after AL (AHR = 0.20, 95% CI 0.10-0.41, p < 0.001) and at day 63 after DP (AHR = 0.08, 95% CI 0.01-0.70, p = 0.0233). Results were limited by follow-up of patients to 63 days or less and nonrandomised treatment groups.
Conclusions: In this study, we observed the risk of P. vivax recurrence at day 42 to be significantly lower following treatment with DP compared with AL, reflecting the longer period of post-treatment prophylaxis; this risk was reduced substantially by coadministration with PQ. We found that delaying P. vivax recurrence was associated with a small but significant improvement in haemoglobin. These results highlight the benefits of PQ radical cure and also the provision of blood-stage antimalarial agents with prolonged post-treatment prophylaxis.
Conflict of interest statement
I have read the journal's policy and the authors of this manuscript have the following competing interests: NJW is a member of the Editorial Board of PLOS Medicine.
Figures


References
-
- Tarning J, Thana P, Phyo AP, Lwin KM, Hanpithakpong W, Ashley EA, et al. Population Pharmacokinetics and Antimalarial Pharmacodynamics of Piperaquine in Patients With Plasmodium vivax Malaria in Thailand. CPT Pharmacometrics Syst Pharmacol. 2014;3:e132 Epub 2014/08/28. 10.1038/psp.2014.29 - DOI - PMC - PubMed
-
- Sumawinata IW, Bernadeta, Leksana B, Sutamihardja A, Purnomo, Subianto B, et al. Very high risk of therapeutic failure with chloroquine for uncomplicated Plasmodium falciparum and P. vivax malaria in Indonesian Papua. Am J Trop Med Hyg. 2003;68(4):416–20. Epub 2003/07/24. . - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

