Synthetic Biology Enables Programmable Cell-Based Biosensors
- PMID: 31585026
- PMCID: PMC7004036
- DOI: 10.1002/cphc.201900739
Synthetic Biology Enables Programmable Cell-Based Biosensors
Abstract
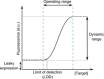
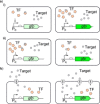
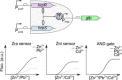
Cell-based biosensors offer cheap, portable and simple methods of detecting molecules of interest but have yet to be truly adopted commercially. Issues with their performance and specificity initially slowed the development of cell-based biosensors. With the development of rational approaches to tune response curves, the performance of biosensors has rapidly improved and there are now many biosensors capable of sensing with the required performance. This has stimulated an increased interest in biosensors and their commercial potential. However the reliability, long term stability and biosecurity of these sensors are still barriers to commercial application and public acceptance. Research into overcoming these issues remains active. Here we present the state-of-the-art tools offered by synthetic biology to allow construction of cell-based biosensors with customisable performance to meet the real world requirements in terms of sensitivity and dynamic range and discuss the research progress to overcome the challenges in terms of the sensor stability and biosecurity fears.
Keywords: cell-based biosensor; genetic circuits; rational approaches; response curve; synthetic biology.
©2019 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Wan X., Volpetti F., Petrova E., French C., Maerkl S. J., Wang B., Nat. Chem. Biol. 2019, 15, 540–548. - PubMed
-
- Siegfried K., Endes C., Bhuiyan A. F. M. K., Kuppardt A., Mattusch J., van der Meer J. R., Chatzinotas A., Harms H., Environ. Sci. Technol. 2012, 46, 3281–3287. - PubMed
-
- Polizzi K. M., Kontoravdi C., Curr. Opin. Biotechnol. 2015, 31, 50–56. - PubMed
-
- Kumari A., Pasini P., Daunert S., Anal. Bioanal. Chem. 2008, 391, 1619–1627. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

