Formyl peptide receptor 2 regulates monocyte recruitment to promote intestinal mucosal wound repair
- PMID: 31585047
- PMCID: PMC6894067
- DOI: 10.1096/fj.201901163R
Formyl peptide receptor 2 regulates monocyte recruitment to promote intestinal mucosal wound repair
Abstract
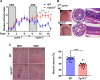
Mucosal wound repair is coordinated by dynamic crosstalk between endogenous and exogenous mediators and specific receptors on epithelial cells and infiltrating immune cells. One class of such receptor-ligand pairs involves formyl peptide receptors (FPRs) that have been shown to influence inflammatory response and repair. Here we explored the role of murine Fpr2/3, an ortholog of human FPR2/receptor for lipoxin A4 (ALX), in orchestrating intestinal mucosal repair. Compared with wild-type (WT) mice, Fpr2/3-/- mice exhibited delayed recovery from acute experimental colitis and perturbed repair after biopsy-induced colonic mucosal injury. Decreased numbers of infiltrating monocytes were observed in healing wounds from Fpr2/3-/- mice compared with WT animals. Bone marrow transplant experiments revealed that Fpr2/3-/- monocytes showed a competitive disadvantage when infiltrating colonic wounds. Moreover, Fpr2/3-/- monocytes were defective in chemotactic responses to the chemokine CC chemokine ligand (CCL)20, which is up-regulated during early phases of inflammation. Analysis of Fpr2/3-/- monocytes revealed altered expression of the CCL20 receptor CC chemokine receptor (CCR)6, suggesting that Fpr2/3 regulates CCL20-CCR6-mediated monocyte chemotaxis to sites of mucosal injury in the gut. These findings demonstrate an important contribution of Fpr2/3 in facilitating monocyte recruitment to sites of mucosal injury to influence wound repair.-Birkl, D., O'Leary, M. N., Quiros, M., Azcutia, V., Schaller, M., Reed, M., Nishio, H., Keeney, J., Neish, A. S., Lukacs, N. W., Parkos, C. A., Nusrat, A. Formyl peptide receptor 2 regulates monocyte recruitment to promote intestinal mucosal wound repair.
Keywords: FPR2; GPCRs; epithelium; inflammation; inflammatory bowel disease.
Conflict of interest statement
The authors thank Prof. Mauro Perretti (Queen Mary University of London London, United Kingdom) for providing the
Figures




References
-
- Leoni G., Neumann P. A., Kamaly N., Quiros M., Nishio H., Jones H. R., Sumagin R., Hilgarth R. S., Alam A., Fredman G., Argyris I., Rijcken E., Kusters D., Reutelingsperger C., Perretti M., Parkos C. A., Farokhzad O. C., Neish A. S., Nusrat A. (2015) Annexin A1-containing extracellular vesicles and polymeric nanoparticles promote epithelial wound repair. J. Clin. Invest. 125, 1215–1227 - PMC - PubMed
-
- Nathan C. (2002) Points of control in inflammation. Nature 420, 846–852 - PubMed
-
- Serhan C. N., Savill J. (2005) Resolution of inflammation: the beginning programs the end. Nat. Immunol. 6, 1191–1197 - PubMed
-
- Fullerton J. N., Gilroy D. W. (2016) Resolution of inflammation: a new therapeutic frontier. Nat. Rev. Drug Discov. 15, 551–567 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

