An Allosteric Anti-tryptase Antibody for the Treatment of Mast Cell-Mediated Severe Asthma
- PMID: 31585081
- PMCID: PMC8403554
- DOI: 10.1016/j.cell.2019.09.009
An Allosteric Anti-tryptase Antibody for the Treatment of Mast Cell-Mediated Severe Asthma
Erratum in
-
An Allosteric Anti-tryptase Antibody for the Treatment of Mast Cell-Mediated Severe Asthma.Cell. 2020 Jan 23;180(2):406. doi: 10.1016/j.cell.2020.01.003. Cell. 2020. PMID: 31978350 No abstract available.
Abstract
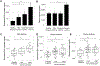
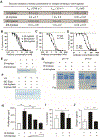
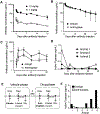
Severe asthma patients with low type 2 inflammation derive less clinical benefit from therapies targeting type 2 cytokines and represent an unmet need. We show that mast cell tryptase is elevated in severe asthma patients independent of type 2 biomarker status. Active β-tryptase allele count correlates with blood tryptase levels, and asthma patients carrying more active alleles benefit less from anti-IgE treatment. We generated a noncompetitive inhibitory antibody against human β-tryptase, which dissociates active tetramers into inactive monomers. A 2.15 Å crystal structure of a β-tryptase/antibody complex coupled with biochemical studies reveal the molecular basis for allosteric destabilization of small and large interfaces required for tetramerization. This anti-tryptase antibody potently blocks tryptase enzymatic activity in a humanized mouse model, reducing IgE-mediated systemic anaphylaxis, and inhibits airway tryptase in Ascaris-sensitized cynomolgus monkeys with favorable pharmacokinetics. These data provide a foundation for developing anti-tryptase as a clinical therapy for severe asthma.
Keywords: allosteric protease inhibitor; anti-IgE; anti-tryptase; antibody engineering; asthma; mast cell; non-type 2 asthma; serine protease; tryptase; tryptase genetics.
Copyright © 2019 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests
All authors except N.N.T., L.B.S., M.B., P.G.W., J.V.F., R.A., and G.H.C. are current or past employees of Genentech, Inc., a member of the Roche group, and may hold Roche stock or stock options. A patent application, entitled “anti-tryptase antibodies, compositions thereof, and uses thereof”, relating to the subject matter of this manuscript, has been filed by Genentech Inc. L.B.S. and G.H.C. are consultants for Genentech for anti-tryptase antibody development.
Figures




References
-
- Alter SC, Kramps JA, Janoff A, and Schwartz LB (1990). Interactions of human mast cell tryptase with biological protease inhibitors. Archives of biochemistry and biophysics 276, 26–31. - PubMed
-
- Babina M, Guhl S, Artuc M, Trivedi NN, and Zuberbier T (2016). Phenotypic variability in human skin mast cells. Exp Dermatol 25, 434–439. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

