De Novo Pathogenic Variants in N-cadherin Cause a Syndromic Neurodevelopmental Disorder with Corpus Collosum, Axon, Cardiac, Ocular, and Genital Defects
- PMID: 31585109
- PMCID: PMC6817525
- DOI: 10.1016/j.ajhg.2019.09.005
De Novo Pathogenic Variants in N-cadherin Cause a Syndromic Neurodevelopmental Disorder with Corpus Collosum, Axon, Cardiac, Ocular, and Genital Defects
Abstract
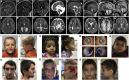
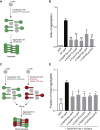
Cadherins constitute a family of transmembrane proteins that mediate calcium-dependent cell-cell adhesion. The extracellular domain of cadherins consists of extracellular cadherin (EC) domains, separated by calcium binding sites. The EC interacts with other cadherin molecules in cis and in trans to mechanically hold apposing cell surfaces together. CDH2 encodes N-cadherin, whose essential roles in neural development include neuronal migration and axon pathfinding. However, CDH2 has not yet been linked to a Mendelian neurodevelopmental disorder. Here, we report de novo heterozygous pathogenic variants (seven missense, two frameshift) in CDH2 in nine individuals with a syndromic neurodevelopmental disorder characterized by global developmental delay and/or intellectual disability, variable axon pathfinding defects (corpus callosum agenesis or hypoplasia, mirror movements, Duane anomaly), and ocular, cardiac, and genital anomalies. All seven missense variants (c.1057G>A [p.Asp353Asn]; c.1789G>A [p.Asp597Asn]; c.1789G>T [p.Asp597Tyr]; c.1802A>C [p.Asn601Thr]; c.1839C>G [p.Cys613Trp]; c.1880A>G [p.Asp627Gly]; c.2027A>G [p.Tyr676Cys]) result in substitution of highly conserved residues, and six of seven cluster within EC domains 4 and 5. Four of the substitutions affect the calcium-binding site in the EC4-EC5 interdomain. We show that cells expressing these variants in the EC4-EC5 domains have a defect in cell-cell adhesion; this defect includes impaired binding in trans with N-cadherin-WT expressed on apposing cells. The two frameshift variants (c.2563_2564delCT [p.Leu855Valfs∗4]; c.2564_2567dupTGTT [p.Leu856Phefs∗5]) are predicted to lead to a truncated cytoplasmic domain. Our study demonstrates that de novo heterozygous variants in CDH2 impair the adhesive activity of N-cadherin, resulting in a multisystemic developmental disorder, that could be named ACOG syndrome (agenesis of corpus callosum, axon pathfinding, cardiac, ocular, and genital defects).
Keywords: ACOG; CDH2; N-cadherin; cardiac defects; cell-cell adhesion; corpus callosum; eye defects; genital defects; intellectual disability.
Copyright © 2019 American Society of Human Genetics. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
K.McW. and A.B. are employees of GeneDx, Inc.
Figures

References
-
- Wickström S.A., Niessen C.M. Cell adhesion and mechanics as drivers of tissue organization and differentiation: local cues for large scale organization. Curr. Opin. Cell Biol. 2018;54:89–97. - PubMed
-
- Priest A.V., Shafraz O., Sivasankar S. Biophysical basis of cadherin mediated cell-cell adhesion. Exp. Cell Res. 2017;358:10–13. - PubMed
-
- Tiwari P., Mrigwani A., Kaur H., Kaila P., Kumar R., Guptasarma P. Structural-mechanical and biochemical functions of classical cadherins at cellular junctions: A review and some hypotheses. Adv. Exp. Med. Biol. 2018;1112:107–138. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

