Mouse Models of Human Gastric Cancer Subtypes With Stomach-Specific CreERT2-Mediated Pathway Alterations
- PMID: 31585123
- PMCID: PMC6902245
- DOI: 10.1053/j.gastro.2019.09.026
Mouse Models of Human Gastric Cancer Subtypes With Stomach-Specific CreERT2-Mediated Pathway Alterations
Abstract
Background & aims: Patterns of genetic alterations characterize different molecular subtypes of human gastric cancer. We aimed to establish mouse models of these subtypes.
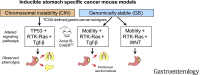
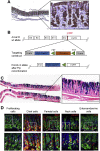
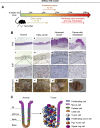
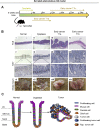
Methods: We searched databases to identify genes with unique expression in the stomach epithelium, resulting in the identification of Anxa10. We generated mice with tamoxifen-inducible Cre recombinase (CreERT2) in the Anxa10 gene locus. We created 3 mouse models with alterations in pathways that characterize the chromosomal instability (CIN) and the genomically stable (GS) subtypes of human gastric cancer: Anxa10-CreERT2;KrasG12D/+;Tp53R172H/+;Smad4fl/f (CIN mice), Anxa10-CreERT2;Cdh1fl/fl;KrasG12D/+;Smad4fl/fl (GS-TGBF mice), and Anxa10-CreERT2;Cdh1fl/fl;KrasG12D/+;Apcfl/fl (GS-Wnt mice). We analyzed tumors that developed in these mice by histology for cell types and metastatic potential. We derived organoids from the tumors and tested their response to chemotherapeutic agents and the epithelial growth factor receptor signaling pathway inhibitor trametinib.
Results: The gastric tumors from the CIN mice had an invasive phenotype and formed liver and lung metastases. The tumor cells had a glandular morphology, similar to human intestinal-type gastric cancer. The gastric tumors from the GS-TGFB mice were poorly differentiated with diffuse morphology and signet ring cells, resembling human diffuse-type gastric cancer. Cells from these tumors were invasive, and mice developed peritoneal carcinomatosis and lung metastases. GS-Wnt mice developed adenomatous tooth-like gastric cancer. Organoids derived from tumors of GS-TGBF and GS-Wnt mice were more resistant to docetaxel, whereas organoids from the CIN tumors were more resistant to trametinib.
Conclusions: Using a stomach-specific CreERT2 system, we created mice that develop tumors with morphologic similarities to subtypes of human gastric cancer. These tumors have different patterns of local growth, metastasis, and response to therapeutic agents. They can be used to study different subtypes of human gastric cancer.
Keywords: Carcinogenesis; Cre/loxP System; Stomach Cancer; Targeted Therapy.
Copyright © 2019 AGA Institute. Published by Elsevier Inc. All rights reserved.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

