Sequential and combination treatments with novel agents in chronic lymphocytic leukemia
- PMID: 31585959
- PMCID: PMC6821614
- DOI: 10.3324/haematol.2018.208603
Sequential and combination treatments with novel agents in chronic lymphocytic leukemia
Abstract
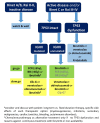
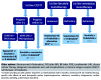
Chemoimmunotherapy has been the standard of care for patients with chronic lymphocytic leukemia for a long time. However, over the last few years, novel agents have produced unprecedented outcomes in treatment-naïve and relapsed/refractory chronic lymphocytic leukemia. With the advent of these targeted agents, treatment options have diversified very considerably and new questions have emerged. For example, it is unclear whether these novel agents should be used as sequential monotherapies until disease progression or whether they should preferably be combined in time-limited treatment regimens aimed at achieving deep and durable remissions. While both approaches yield high response rates and long progression-free and overall survival, it remains challenging to identify patients individually for the optimal concept. This review provides guidance in this decision process by presenting evidence on sequential and combined use of novel agents and discussing the advantages and drawbacks of these two approaches.
Copyright© 2019 Ferrata Storti Foundation.
Figures

References
-
- Eichhorst B, Fink AM, Bahlo J, et al. First-line chemoimmunotherapy with bendamustine and rituximab versus fludarabine, cyclophosphamide, and rituximab in patients with advanced chronic lymphocytic leukaemia (CLL10): an international, open-label, randomised, phase 3, non-inferiority trial. Lancet Oncol. 2016;17(7):928–942. - PubMed
-
- Hallek M, Fischer K, Fingerle-Rowson G, et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet. 2010;376(9747):1164–1174. - PubMed
-
- Hallek M, Cheson BD, Catovsky D, et al. iwCLL guidelines for diagnosis, indications for treatment, response assessment, and supportive management of CLL. Blood. 2018;131(25):2745–2760. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

